(1)
Institut Bergonié, Bordeaux, France
Abstract
Semaphorins constitute a group of signalling proteins originally described in the central nervous system where they are involved in the growth of the axonal cone and in axonal guidance. They have in fact pleiotropic roles during development of multiple organs and can stimulate or inhibit the same function according to the receptors activated in the target tissues. Semaphorin receptors are especially present at the surface of endothelial cells, which implies that they play a role in angiogenesis. Since most semaphorins are membrane integral proteins, the signalling pathways are exclusively implemented in juxtacrine situations. Semaphorins play a major role in cell adhesion and motility, so that the alterations of this signalling pathway contribute to neurodegenerative diseases and cancer.
Only some of the signalling pathways involving semaphorins are presently known from ligand binding to intracellular effectors: we will present them in this chapter. Many things remain to be discovered in the semaphorin field. This chapter is completed by a short presentation of various adhesion molecules, outside the semaphorin field, which are involved in various types of cell–cell and cell–matrix contacts, in addition to those already studied in Chaps. 10 and 11.
11.1 Semaphorins and Semaphorin Receptors
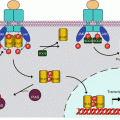
Semaphorins, usually abbreviated as SEMA, constitute in vertebrates a group of 20 proteins distributed in 5 classes, from class 3 to class 7. Those belonging to class 3 are secreted, whereas the other ones are membrane-bound, either through the existence of a single transmembrane domain (semaphorins of classes 4, 5 and 6) or by anchoring with a glycosylphosphatidylinositol membrane lipid (semaphorin 7A). All semaphorins have a characteristic N-terminal domain called the SEMA domain, 500 amino acids long, which consists of a seven-blade β-propeller structure and contains dimerisation and receptor-binding sites. They also contain other specific domains (Fig. 11.1). Semaphorins operate as homodimers associating two polypeptidic chains bound by a disulphide bridge.
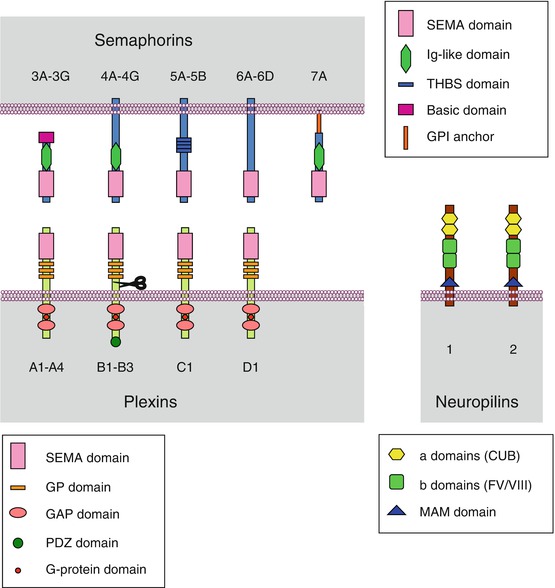

Fig. 11.1
General structure of semaphorins, plexins and neuropilins. Semaphorins (SEMAs) are a family of 21 proteins distributed in 5 subfamilies, from semaphorins 3 to semaphorins 7. They are either secreted (semaphorins 3) or bound to the plasma membrane. Membrane insertion is obtained either by a transmembrane domain (semaphorins 4, 5 and 6) or anchoring via a glycosylphosphatidylinositol (GPI) group (semaphorins 7). All semaphorins contain a SEMA domain allowing receptor binding and dimerisation and, according to the class they belong to, an immunoglobulin-like (IgG) domain, a thrombospondin-like (THBS) domain or a basic domain. Plexins (PLXNs) are a family of 9 proteins distributed in 4 subfamilies, from plexins A to plexins D. These are transmembrane proteins equipped, on the extracellular side, with a SEMA domain and glycine- and proline-rich (GP) domains and, on the intracellular side, with a small G-protein binding domain, a GAP (GTPase activating protein) domain and, for plexins B, a PDZ (postsynaptic density-95, disc-large and zonula occludens) domain and a GEF (guanyl nucleotide exchange factor) domain. Neuropilins (NRPs) are a group of 2 proteins, neuropilin 1 and neuropilin 2. These are transmembrane proteins, with a very short intracellular domain and, on the extracellular side, two domains a for complement binding (CUB, complement protein, urchin embryonic growth factor and bone morphogenic protein 1), two domains b homologous to the coagulation factor V/VIII and a domain c called MAM (meprin, A5 protein, μ protein tyrosine phosphatase), involved in neuropilin dimerisation and interactions with other receptors
The semaphorin receptors of membrane-bound semaphorins (classes 4–7) and semaphorin 3E are the plexins (PLXNs): plexins A for semaphorins 6, plexins B for semaphorins 4 (except semaphorin 4A) and semaphorins 5, plexin C1 for semaphorin 7A and plexin D1 for semaphorins 3E and 4A. A given semaphorin can bind several plexin receptors, and a given plexin can bind several semaphorin ligands (Table 11.1). Secreted semaphorins (class 3, but with the exception of semaphorin 3E) use neuropilins (NRPs) as primary receptors, together with plexins A, which are responsible for signal transduction. In lymphocytes, SEMA4A also uses TIM2 (T-cell immunoglobulin and mucin domain-containing protein), as a receptor or coreceptor, while SEMA4D can use CD72, a membrane lectin expressed in B-cells. Diverse membrane proteins may be associated to semaphorin reception: tyrosine kinase receptors (TKR, Chap. 1), integrins (Chap. 10), through the interaction of a RGD motif borne by SEMA7A, as well as TREM2 (triggering receptor expressed on myeloid cells 2) and DAP12 (DNAX-activation protein 12), a TRK-binding protein encoded by the gene TYROBP (TYRO protein tyrosine kinase binding protein). Proteoglycans with heparan sulphate (HSPG) or chondroitin sulphate (CSPG) moieties are also associated with several semaphorin receptors.
Table 11.1
Combinatorial pattern of semaphorins and semaphorin receptors
Receptor | Ligand | Coreceptor |
|---|---|---|
PLXNA1 | SEMA6C, SEMA6D | |
PLXNA2 | SEMA6A, SEMA6B | |
PLXNA3 | ||
PLXNA4 | SEMA6A, SEMA6B | |
PLXNB1 | SEMA4D | CD72 |
PLXNB2 | SEMA4A, SEMA4C, SEMA4D | |
PLXNB3 | SEMA5A, SEMA5B | SYN3 |
PLXNC1 | SEMA7A | Integrin α1β1 |
PLXND1 | SEMA3E | NRP1, NRP2 |
SEMA4A | TIM2a | |
NRP1 | SEMA3A, SEMA3B, SEMA3C, SEMA3D | PLXNA1-PLXNA4 |
NRP2 | SEMA3B, SEMA3C, SEMA3D, SEMA3F, SEMA3G |
Plexins, which appear as the main semaphorin receptors, constitute a family of 9 proteins distributed in 4 classes (A to D). These proteins display a single transmembrane domain and, on the intracytoplasmic side, a binding domain to small G-proteins of the RHO subfamily such as RHOD, RND1 or RAC1, and they exert on these small G-proteins a GTPase stimulating activity (GAP). Plexins B present in addition, on the C-terminal side, a PDZ (postsynaptic density 95/disc-large/zona occludens) domain for binding a GDP–GTP exchange factor (GEF) for small G-proteins. The extracellular part of plexins contain a SEMA domain involved in semaphorin binding, and class B plexins have in addition a proteolytic domain similar to that of the subtilisin protein convertase, furin (Fig. 11.1). Due to the dimeric structure of the active ligands, plexins are thought to also act as dimers.
Neuropilins (2 proteins in mammals) are the primary receptors of class 3 semaphorins (excepted SEMA3E), but signalling requires the intervention of a class A plexin. They contain two domains for complement binding (CUB [complement protein, urchin embryonic growth factor and bone morphogenic protein 1]), two domains homologous to the coagulation factor V/VIII and a domain called MAM (meprin, A5 protein, μ protein tyrosine phosphatase), which is important for their dimerisation and their interaction with other receptors. Their intracellular domain is very short and does not seem able of signal transduction, which explains the requirement of a plexin molecule as receptor. Neuropilins can also be used as coreceptors for some forms of VEGF (vascular endothelial growth factor) (Chap. 1) and they interact with VEGF receptors. They may also interact with other TKR, such as MET, FGFR2 and PDGFRB, and serve as coreceptors to several growth factors (Chap. 1). Furthermore, they interact with adhesion factors of the CAM (cell adhesion molecules) family and integrins (Chap. 10).
11.2 Semaphorin-Induced Signal Transmission
The diversity of semaphorins and semaphorin receptors explains why the signalling pathways they activate are only partially known. Only the pathways originating from semaphorins 3A and 4D have been deciphered and will be presented here as representative examples. It appears that the different complexes semaphorin–plexin can each activate several transduction pathways involving various systems: small G-proteins, cytoplasmic tyrosine kinases, integrins, etc. The action of semaphorins on cytoskeleton, cell adhesion and motility is pleiotropic and certainly redundant.
11.2.1 Semaphorin 3A Signalling
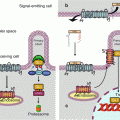
The reception complex NRP1–PLXNA1, at the basal inactive state, is associated to a protein called FARP2 (FERM, RHO-GEF and pleckstrin domain protein 2) with GEF (guanine nucleotide exchange factor) activity (Fig. 11.2a). When bound to SEMA3A, the reception complex releases FARP2, unmasking its GEF activity and allowing it to activate RAC1, a small G-protein, through GDP–GTP exchange. RAC1 can then sequentially activate the serine/threonine kinases PAK1 (p21-activated kinase 1) and LIMK1 (LIM domain kinase 1) and cofilin (CFL), a protein controlling actin polymerisation (Fig. 11.2b). RAC1 facilitates also the association of RND1, another small G-protein of the RHO subfamily, with plexin A1, which stimulates the GAP activity of the plexin. The target of this action of plexin A1 is RRAS, another small G-protein also involved in integrin signalling (Fig. 11.2c). This results in the inactivation of integrin β1 subunit, inducing the detachment of cells from the extracellular matrix. The interaction of RND1 with plexin A can be inhibited by another small G-protein, RHOD, which can bind the same site of plexin A1.
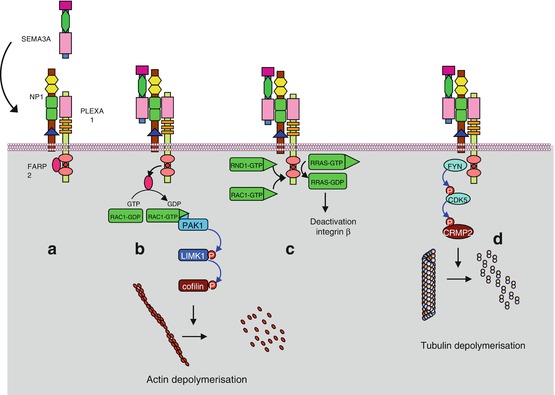

Fig. 11.2
Semaphorin 3A signalling pathway. (a) SEMA3A recognises, on the target cell, an inactive reception complex constituted of NPL1 and PLXNA1. This plexin is bound to protein FARP2 (FERM, RHO-GEF and pleckstrin domain protein 2), which is a guanyl nucleotide exchange factor (GEF). (b) The interaction of SEMA3A with the reception complex induces the release of FARP2, which can exchange the GDP bound to the small G-protein RAC1 against GTP; RAC1 is thus activated and induces a cascade of kinases, PAK1 and LIMK1, leading to the activation of cofilin (CFL), which depolymerises the actin cytoskeleton. (c) RAC1 and RND, other small G-proteins of the RHO subfamily, activate the GAP (GTPase activating protein) function of PLXNA1, which deactivates another small G-protein, RRAS. This induces the deactivation of integrin β1 subunit and the detachment of the cell from the extracellular matrix. (d) Once activated, PLXA1 can in turn activate cytoplasmic tyrosine kinases such as FYN, FES or FER. They recruit in turn and phosphorylate the serine/threonine kinase CDK5, allowing thus the activation of CRMP2 (collapsin response mediator protein, gene DPYSL2, dihydropyrimidinase-like 2), a microtubule-depolymerising agent
Upon activation by SEMA3A, the NRP–PLXNA1 reception complex can activate cytoplasmic tyrosine kinases such as FYN, FES or FER. This activation allows the phosphorylation of plexin A1 by CDK5 (cyclin-dependent kinase 5), which induces the recruitment of a protein called CRMP2 (collapsin response mediator protein 2) (gene DPYSL2 [dihydropyrimidinase-like 2]) to be phosphorylated (Fig. 11.2d). CRMPs act as microtubule-depolymerising agents and are thus mediators of SEMA3A-induced effects on the cytoskeleton.
11.2.2 Semaphorin 4D Signalling
Plexin B1, the semaphorin 4D receptor, is dimeric in the absence of signal. Binding SEMA4D induces the activation of small G-proteins of the RHO subfamily (RHOD, RND1, RAC1), giving similar effects to those elicited by SEMA3A on the NRP1–PLXNA1 reception complex. However, SEMA4D may also have opposite effects: the activation of its receptor may induce, on the one hand, the activation of a RHO-GAP protein (and therefore RHO deactivation); and on the other hand the activation of a RHO-GEF protein (and therefore RHO activation).
11.3 Oncogenic Alterations and Pharmacological Targets
Semaphorins play a major role in cell adhesion and motility and consequently in invasion, metastasis, angiogenesis and immune processes. Semaphorin signalling can induce opposite effects as a function of the ligand–receptor system involved and may give positive or negative instructions concerning cancer cell growth and metastatic dissemination. Semaphorins 3B and 3F are considered as tumour suppressors; the loss of function of SEMA3B, related to promoter hypermethylation or gene polymorphism, is frequently found in non-small-cell lung cancers. This semaphorin has experimentally an inhibitor effect on cell growth. Similar observations have been made on SEMA3F, which displays antiangiogenic properties and inhibits cell attachment to the extracellular matrix; this semaphorin could be, therefore, antimetastatic, as SEMA3A. Semaphorins 3C and 3E have been shown in contrast to favour angiogenesis and consequently tumour progression. SEMA4D, which induces the activation of the MET and ERBB2 tyrosine kinase receptors, via PLXNB1, favours tumour invasiveness; it has also been shown to display proangiogenic properties; the same is true for the couple SEMA5B–PLXNB3.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree