Keywords
Oncolytic virus, adenovirus, chemotherapy, radiotherapy, molecular targeted therapy, clinical trial, combined therapy
Introduction
Gene and vector-based molecular therapies for cancer encompass a wide range of treatment types that use genetic material to modify cancer cells and/or surrounding tissues to exhibit antitumor properties. One of the most common approaches from the concept of gene therapy is the introduction of foreign therapeutic genes, such as the p53 gene, into target cells . However, failure to achieve complete viral delivery to the majority of the cells within a clinically presented three-dimensional solid tumor mass is a limitation of this approach. To overcome these clinical limitations of cancer gene therapy based on replication-defective virus vectors, the use of oncolytic virus vectors for selective tumor cell destruction has rapidly emerged as a promising area of molecular therapy for cancer. Infection with oncolytic viruses results in viral replication and ultimately the release of viral progeny, which facilitates viral spread to neighboring tumor cells and enhances distribution throughout the targeted tumor bed. Therefore, oncolytic viruses can be used as anticancer agents by genetic engineering to replicate selectively in cancer cells while remaining innocuous to normal tissues .
Moreover, in addition to their own oncolytic capability, these oncolytic viruses can be engineered to facilitate gene delivery to virus-infected tumor cells to produce therapeutic effects such as apoptosis, cytokine secretion, and antitumor immune responses prior to the induction of oncolytic cell death . Indeed, many types of oncolytic viruses, such as herpes simplex virus , adenovirus , Newcastle disease virus , reovirus , vaccinia virus , and Sindbis virus , have been developed, and some of these viruses are in human trials or entering human trials . Among these viruses, adenovirus, especially human type 5 adenovirus (Ad5), is one of the most popular and familiar types of viruses because the genetic information and the biology of natural infection in humans are well characterized and because it has a long history of being used as a gene delivery vector . Indeed, the mutation-based oncolytic adenovirus H101 was approved in China for the treatment of patients with head and neck cancer in 2005 ; so far, H101 is the first and only oncolytic virus to be approved as a drug in humans. The approval of H101 brings hope that oncolytic adenoviruses will be available in humans after the large setback that occurred in 1999 with the death of Jesse Gelsinger in a clinical trial for gene therapy with an adenovirus vector .
Adenovirus (90–100 nm in size) is a nonenveloped virus with a double-stranded linear DNA genome. Most of the 57 adenovirus serotypes in humans are pathogens responsible for common upper respiratory infections. Adenovirus entry into the host cell is mainly initiated by the interaction of the adenovirus fiber knob and the cell receptor, which is the coxsackievirus–adenovirus receptor (CAR) for most types of adenovirus. Then, the adenovirus is taken up into cells by clathrin-dependent endocytosis, followed by the nuclear transportation of viral DNA via the nuclear pore after escape from the endosome . Adenoviral replication consists of two phases, an early phase and a late phase. In the early phase, two adenoviral early gene products, E1A (which binds to the retinoblastoma (Rb) protein) and E1B (which binds to p53), immortalize the host cells by inhibiting these two tumor suppressor genes and creating a proper environment for viral replication. In the late phase, adenovirus produces sufficient structural proteins to package the replicated genome. Once adenoviral progenies are successfully created, these viruses are released from the cells by inducing cell lysis, and the released viruses spread to neighboring cells. Replication-defective adenovirus is modified to be unable to replicate after cellular uptake by removal of the E1 region, and replication-competent (oncolytic) adenovirus is modified to replicate and induce cell lysis under specific conditions only by the deletion or recombination of the E1 region.
Conventional chemotherapy, radiotherapy, and surgery are still the core strategies for cancer treatment, whereas several molecular targeted agents such as inhibitors of the epidermal growth factor receptor (EGFR), the HER2 receptor, and the vascular endothelial growth factor (VEGF) have emerged as novel treatment methods. It is necessary to examine the combination efficacy of oncolytic adenoviruses with these other treatments and to analyze the mechanisms of any synergistic effects that might occur. We first discuss the development of oncolytic adenoviruses, especially the ones that have been tested in clinical trials. Then, we introduce the preclinical and clinical studies of the efficacy of oncolytic adenoviruses in combination with chemotherapy, radiotherapy, or molecular targeted agents.
Oncolytic Adenoviruses Tested in Clinical Trials
Many oncolytic adenoviruses have been developed, and some of them have been tested in clinical trials based on promising preclinical data. To be used in humans, drugs must be safe as well as effective. Oncolytic adenovirus is considered to be safe compared to other types of viruses because adenovirus is a genetically stable and nonintegrating virus, unlike retroviruses, and it causes only mild, common diseases . However, because the use of adenovirus in humans caused a patient’s death in a clinical trial in 1999 , oncolytic adenoviruses must be fully tested in clinical trials for safety as well as efficacy. Here, we discuss several representative oncolytic adenoviruses that have been tested in clinical trials ( Table 12.1 ).
| Mechanism for Tumor Selectivity | Name | Description |
|---|---|---|
| Gene deletion | ||
| E1B55 kDa gene | ONYX-015 H101 | Inability of inhibiting p53 pathway |
| E1A 24 base pair |
| Inability of inhibiting Rb/p16 pathway |
| Promoter | ||
| PSA | CG7870 | Selective replication in PSA-positive prostate cancers |
| E2F-1 | ICOVIR-7 CG0070 | Selective replication in Rb pathway-defective cancers |
| hTERT | KH901 Telomelysin | Selective replication in hTERT-activated cells (which means most types of cancers) |
ONYX-015 (also known as dl1520) is a pioneer in the field of oncolytic adenovirus and was first tested in clinical settings in 1996. Many studies of ONYX-015 have been published since 1986 when it was first reported. ONYX-015 is characterized by the lack of the E1B-55kDa gene, which blocks p53 function . p53 protein plays critical roles in cell growth regulation, some of which are related to cell cycle arrest and apoptosis. After virus infection, p53 induces either G 1 growth arrest via several cyclin-dependent kinases or apoptosis by activating the caspase cascade and bax to avoid viral propagation . However, wild-type adenovirus can replicate even in cells that have wild-type p53 because it produces E1B-55kDa protein that inactivates p53. On the other hand, ONYX-015, E1B-55kDa-deleted adenovirus, is supposed to replicate only in p53-deleted or -mutated cancer cells while being unable to replicate in p53 wild-type cells. Based on this rationale, ONYX-015 was originally supposed to be sensitive only against p53-deleted or -mutated cancer cells, although effectiveness against p53 wild-type cancer cells was later reported . ONYX-015 was extensively tested in clinical trials from the late 1990s to the early 2000s. Results from the clinical trials indicated that ONYX-015 was safe and selective for cancers, but the therapeutic effect following local or systemic injection was limited . Therefore, further development of ONYX-015 was abandoned in the United States, and the rights were taken over by the Chinese company Shanghai Sunway Biotech. Then, H101, an oncolytic adenovirus that has genetic modifications very similar to those of ONYX-015 (deletion of E1B-55kDa and a part of E3), became the world’s first oncolytic virus to be approved for human use in 2005 when China’s State Food and Drug Administration approved it as a commercial drug for head and neck squamous cell carcinoma based on good efficacy observed in a clinical trial conducted in China . However, some doubts have been raised concerning the fact that in this phase III clinical trial, the efficacy was determined on the basis of the response rate instead of the patient survival, which may be better for the final judgment of a treatment’s efficacy.
Ad5-D24 is an oncolytic adenovirus that carries a 24-base pair (bp) deletion in the E1A region responsible for binding Rb protein, an important tumor suppressor gene that is impaired in several major malignant tumors in humans . One of the E1A functions is disruption of the interaction between Rb and E2F proteins . E2F proteins are sequestered by Rb until progression of the cell cycle from G 1 to S phase, resulting in E2F-dependent cellular gene transcription. Interaction of the adenoviral E1A protein with Rb protein results in the release of E2F from preexisting cellular E2F–Rb complexes. This free E2F protein activates the adenoviral E2 promoter and several cell cycle-related genes, which helps to create a suitable environment for viral replication . Ad5-D24 that produces mutant E1A protein is supposed to be unable to replicate in normal cells, which have normal Rb and are arrested in G 0 phase, while being able to replicate in replicating cells such as malignant tumors because of their disrupted Rb function. Ad5-D24-RGD is a derivative of Ad5-D24, whose fiber region is modified by incorporating an integrin binding RGD-4C motif, allowing CAR-independent infection of cancer cells . Ad5-D24-GMCSF is another derivative of Ad5-D24 that was developed from the standpoint of antitumoral immunity. Granulocyte–macrophage colony-stimulating factor (GM-CSF) is a cytokine that can produce antitumor effects via natural killer cells and tumor-specific cytotoxic T cells. Thus, Ad5-D24-GMCSF is designed to produce more potent immune activity against tumor tissues. The safety and efficacy of Ad5-D24-RGD, Ad5-D24-GMCSF, and the combined Ad5-D24-RGD-GMCSF were evaluated in a clinical study in Finland . A serotype 5/3 chimeric oncolytic adenovirus expressing GM-CSF, Ad5/3-D24-GMCSF (also known as CGTG-102), was also tested in clinical settings in Finland; the intratumoral and intravenous administration of CGTG-102 to 21 patients with advanced solid tumors was well tolerated and showed promising signs of efficacy . ICOVIR-7 is an oncolytic adenovirus designed to focus on the degradation of the Rb/p16 pathway in tumor cells . ICOVIR-7 has a basic construct of Ad5-D24 and is designed to increase the E2F dependency of the E1A gene by using the modified E2F-1 promoter to control E1A expression. A clinical trial to test the safety of ICOVIR-7 intratumoral injection in 21 patients with solid tumors was conducted in Finland, and the results proved that ICOVIR-7 treatment was safe and potentially effective .
H103 is a recombinant oncolytic type 2 adenovirus that overexpresses 70-kDa heat shock protein (HSP70). H103 was developed to maximize the antitumor immune response, which appeared to be important in the H101 clinical trials. In these clinical trials, the patients who developed a fever after H101 intratumoral injection tended to show stronger antitumor activity not only in the locally injected tumor but also in distant metastatic tumors that were not injected than the patients without fever . H103 is expected to produce strong antitumor effects via the immune responses stimulated by the overexpression of HSP70, in addition to the oncolytic activity of the virus. In a phase I clinical trial of intratumoral injection of H103 in 27 advanced solid tumor patients in China, the treatment was well tolerated and displayed encouraging antitumor activity .
Ad5-CD/TK rep is an E1B-attenuated replication-competent adenovirus that delivers a pair of therapeutic suicide genes to tumors . Suicide gene therapy is an approach that involves the tumor-targeted delivery of genes expressing metabolic enzymes that convert systemically delivered innocuous prodrugs into toxic metabolites. Escherichia coli cytosine deaminase (CD) produces cytotoxic effects by converting 5-fluorocytosine to its toxic metabolite, 5-fluorouracil (5-FU), and herpes simplex virus type 1 thymidine kinase (HSV-1 TK) phosphorylates ganciclovir (GCV) to a nucleotide analog that inhibits DNA synthesis . A phase I clinical study of Ad5-CD/TK rep was the first gene therapy trial in which a replication-competent virus was used for therapeutic gene delivery to humans . In this clinical study, Ad-CD/TK rep was intratumorally injected into 16 prostate cancer patients, and the results showed that Ad-CD/TK rep could be safely applied to humans and that it showed signs of biological activity. Ad5-yCD/ mut TK SR39 rep -ADP is a second-generation adenovirus that contains two improvements over the parental Ad5-CD/TK rep . One improvement is that Ad5-yCD/ mut TK SR39 rep -ADP contains a fusion gene of a yeast cytosine deaminase (instead of a bacterial one) and mutant SR39 herpes simplex virus thymidine kinase (instead of a wild-type one) in the E1 region, and the other improvement is that Ad5-yCD/ mut TK SR39 rep -ADP contains the Ad5 adenoviral death protein (ADP) gene in the E3 region. Moreover, Ad5-yCD/ mut TK SR39 rep -hNIS contains the human sodium iodide symporter (hNIS) in the E3 region as a reporter gene to allow noninvasive monitoring of gene expression in humans by positron emission tomography or single-photon emission computed tomography .
CG7870 (formally known as CV787) is a prostate-specific replication-competent adenovirus that contains the prostate-specific rat probasin promoter to drive the Ad5 E1A gene and the human prostate-specific enhancer/promoter to drive the Ad5 E1B gene . Unlike the previously constructed prostate-specific replication-competent adenoviruses such as CV706 and CV764, the Ad5 E3 region is restored in CG7870 to reduce the immune response to the vector. CG7870 can replicate like wild-type Ad5 in prostate-specific antigen (PSA)-positive cells, but its replication is attenuated 10,000 to 100,000 times in PSA-negative cells. A phase I dose-escalation study of intraprostatic delivery of CV706 for 20 patients with locally recurrent prostate cancer was conducted in the United States, and the results showed that CV706 intraprostatic delivery was safe and possessed clinical activity . Moreover, a single intravenous administration of CG7870 was tested in a phase I clinical trial of 23 patients with hormone-refractory metastatic prostate cancer . In this trial, three therapy-related grade 3 flu-like adverse events were reported, one of which was serious, whereas 5 patients had a decrease in PSA of 25–49%. These results suggest that approaches to increase intravenous delivery to tumors are necessary to enhance the antitumoral efficacy and decrease adverse events.
KH901 is a conditionally replicating oncolytic adenovirus that can selectively replicate in and lyse telomerase-positive tumor cells and express GM-CSF . The replication of KH901 is controlled by human telomerase reverse transcriptase (hTERT) that is modified to include two E2F-1 binding sites. KH901 contains human GM-CSF cDNA in the E3 region so that GM-CSF is expressed in cancers to stimulate the immune response. In a phase I clinical study of KH901 intratumoral injection in 23 patients with recurrent head and neck cancer in China, the treatment was shown to be feasible, well tolerated, and associated with biological activity .
CG0070 is a conditionally replicating oncolytic serotype 5 adenovirus in which the viral E1A gene is controlled by the human E2F-1 promoter and human GMCSF cDNA is inserted in the E3 region . CG0070 is designed to replicate and produce GMCSF in Rb pathway-defective tumor cells in which E2F-1 activity is upregulated. The GMCSF then stimulates systemic and tumor-specific immunity. A phase I/II study of intravesical CG0070 administration to 35 patients with superficial bladder cancer after Bacillus Calmette–Guerin failure is ongoing in the United States. So far in this trial, CG0070 has been well tolerated with minimum local and systemic toxicities, and complete responses have occurred in 9 of 13 patients (70%) in which Rb was inactivated.
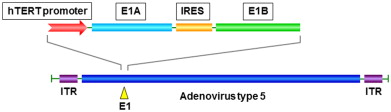
Telomelysin (also known as OBP-301) is an oncolytic adenovirus vector that we previously developed in which the hTERT promoter drives the expression of E1A and E1B genes linked with an internal ribosome entry site ( Figure 12.1 ) . Telomerase is an enzyme that adds DNA sequence repeats (TTAGGG) in the telomere regions. Telomerase activity is found in more than 85% of human cancers , but it is absent in most normal somatic tissues . Human telomerase consists of the human telomerase RNA component (hTERC), the human telomerase-associated protein 1 (hTEP1), and hTERT. The hTERT activity is significantly correlated with the telomerase activity that is enhanced in most tumors , which suggests that the hTERT promoter can be a suitable regulator of adenoviral replication. Indeed, the transcriptional control of E1A expression by the hTERT promoter has been used in several oncolytic adenoviruses, including Telomelysin and the aforementioned KH901 . Based on preclinical studies showing that Telomelysin can selectively kill many types of cancer cells in vitro and in vivo via intracellular viral replication regulated by the hTERT promoter, a dose-escalation phase I clinical trial was conducted in the United States after approval of the U.S. Food and Drug Administration (FDA) in 2006 to determine the safety of Telomelysin intratumoral injection in 16 patients with advanced solid tumors . In this study, a single intratumoral injection of Telomelysin was well tolerated with common grade 1 or 2 local and systemic toxicities, and the treatment showed evidence of antitumor activity.
Combined Therapy of Oncolytic Adenoviruses with Conventional Radiotherapy
Most clinical trials of oncolytic adenoviruses have shown that dramatic antitumor effects are unlikely from monotherapy with oncolytic adenovirus; therefore, combination therapies of oncolytic adenoviruses with other therapeutic modalities must be considered. Radiotherapy is a standard treatment method for a variety of malignant tumors, and more than 50% of cancer patients receive radiotherapy at some point during treatment . Radiation is a local treatment modality like surgery, and it targets not only primary tumors but also regional lymph nodes. Similarly, the main target of oncolytic adenoviruses is the locoregional area because their delivery method is currently limited to intratumoral administration, which suggests that radiation and oncolytic adenoviruses could be a good combination for cancer treatment. Indeed, the combination of oncolytic adenoviruses with radiotherapy is becoming promising as the combined effects are examined and better understood. Many preclinical and clinical studies have shown synergistic antitumor effects of these two treatment methods, indicating the occurrence of either oncolytic virus-mediated sensitization to radiotherapy or radiation-mediated sensitization to oncolytic cell death.
When the combination of ONYX-015 intratumoral injection with radiotherapy was tested in a subcutaneous human malignant glioma xenograft model, the potentiation of radiotherapy by ONYX-015 was observed, although neither increased adenoviral infectivity nor replication by radiation was found . Increased efficacy of radiation therapy following ONYX-015 intratumoral injection was also demonstrated in an anaplastic thyroid carcinoma mouse xenograft model . Treatment with Ad5-D24, Ad5-D24-p53, or Ad5-D24-RGD in combination with radiation in a subcutaneous malignant glioma mouse model demonstrated a significantly high rate of tumor regression as well as long-term survival . Prostate cancer-specific adenoviruses, CV706 and CG7870, in combination with radiation in a prostate cancer xenograft mouse model resulted in the synergistic enhancement of antitumor efficacy with no significant side effects . Ad5-CD/TK rep and Ad5-yCD/ mut TK SR39 rep -ADP, first- and second-generation oncolytic adenoviruses armed with double suicide genes, in combination with radiation were tested in phase I clinical trials in patients with newly diagnosed prostate cancer, and the combination of these oncolytic adenoviruses with radiation demonstrated the potential to improve the outcome of radiotherapy in select patient groups .
The mechanism for the synergistic effects of oncolytic adenoviruses and radiotherapy is not well-established, unlike the synergy between herpes virus and radiation, in which the radiation induces cellular responses that enhance viral replication by increasing cellular GADD34 expression . It has been hypothesized that the increase in viral replication by radiation may be a partial reason for the radiation-mediated enhancement of viral oncolysis, although little information about the underlying molecular mechanisms has been revealed . With regard to the increase in viral uptake into cells, the upregulation of CAR by radiation has been considered the most convincing reason ; however, there have been contradictory reports, one of which is that radiation-mediated upregulation of Dynamin 2 is a main factor for increased viral uptake by endocytosis after radiation .
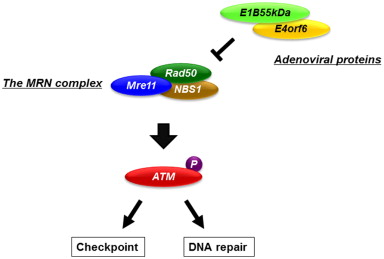
We have proposed a possible mechanism by which oncolytic adenoviruses that contain the E1B-55 kDa gene have the potential to sensitize cancer cells to ionizing radiation . Ionizing radiation produces its cytotoxic effect by targeting DNA molecules and causing DNA double-strand breaks (DSBs). Ataxia–telangiectasia mutated (ATM) protein is a critical signal transducer of the DNA damage response, which leads to DNA repair and the cell cycle checkpoints. Cells that have mutated ATM genes have defects in DNA repair and the cell cycle checkpoint and are hypersensitive to DNA DSBs . The protein complex formed by Mre11, Rad50, and NBS1 (the MRN complex) is quickly stimulated by DNA DSBs and directly activates ATM . The adenoviral E1B-55kDa protein inhibits the function of the MRN complex as well as p53 by cooperating with the adenoviral E4orf6 protein and leading to the proteolytic degradation of these proteins . Thus, oncolytic adenoviruses with the E1B gene can potentiate the effects of radiotherapy by inhibiting DNA repair via the ATM pathway through the E1B-55kDa protein-mediated degradation of the MRN complex ( Figure 12.2 ).
We showed that the telomerase-dependent oncolytic adenovirus Telomelysin, which we developed, and ionizing radiation can potentiate each other against human cancer cells in vitro . Not only does Telomelysin enhance the effects of ionizing radiation through the mechanisms mentioned previously but also ionizing radiation can enhance the antitumor effects of Telomelysin . Ionizing radiation increased CAR expression on the surface of cancer cells, leading to the increased infectivity of Telomelysin, whereas ionizing radiation did not affect the replication efficiency of Telomelysin. Moreover, in vivo studies of Telomelysin intratumoral injection combined with ionizing radiation in subcutaneous (non-small-cell lung cancer and esophageal cancer) or orthotopic (esophageal cancer) xenograft mouse models demonstrated potent antitumor effects.
Combined Therapy of Oncolytic Adenoviruses with Conventional Chemotherapeutic Agents
Chemotherapy is a standard treatment regimen for various cancers, especially advanced cancers that have spread systemically and are difficult to cure by locoregional treatments such as surgical resection and radiotherapy. Chemotherapy is often combined with other treatments as a part of a multidisciplinary treatment. Most chemotherapeutic agents produce cytotoxic effects by targeting rapidly dividing cells, which means that these chemotherapeutic agents kill not only cancer cells but also normal cells that divide rapidly, such as cells of the bone marrow, gastrointestinal tract, and hair, resulting in the side effects of chemotherapy. Because oncolytic adenoviruses possess a high level of tumor specificity and different mechanisms for cytotoxic activity from chemotherapeutic agents, a combination of oncolytic adenoviruses and chemotherapy is expected to produce synergistic antitumor effects. Here, we discuss the combined efficacy of oncolytic adenoviruses with common chemotherapeutic agents, such as antimetabolites (5-FU and gemcitabine), alkylating agents (cisplatin, carboplatin, oxaliplatin, cyclophosphamide, and mitomycin C), taxanes (paclitaxel and docetaxel), and topoisomerase inhibitors (irinotecan, topotecan, doxorubicin, and epirubicin), as reported in preclinical and clinical studies.
Antimetabolites
5-FU is a pyrimidine analog that has been used as a chemotherapeutic agent for various types of cancers for more than 40 years. It produces antitumor effects by inhibiting synthesis of the pyrimidine thymidine, which is a nucleoside required for DNA replication . The combined antitumor effects of systemically administered ONYX-015 with 5-FU were tested in a xenograft mouse model and found to be dramatically greater than the effect of either agent by itself . In this study, six complete responses occurred in mice that received the combination therapy, whereas only one complete response occurred in mice that received only ONYX-015. A phase II clinical trial of a combination of intratumoral ONYX-015 injection with standard intravenous cisplatin and 5-FU chemotherapy in patients with recurrent squamous cell cancer of the head and neck was conducted in the United States. The combined therapy showed a higher rate of successful local control (including complete response) than chemotherapy alone, although it remains to be determined if this enhanced local control will be translated into a prolonged overall survival rate . Further development of the ONYX-015 concept occurred with studies of the closely related H101. A phase III clinical trial of H101 intratumoral injection combined with cisplatin plus 5-FU (PF) or adriamycin plus 5-FU (AF) versus either chemotherapy regimen alone in 123 patients with head and neck or esophageal squamous cell cancer was conducted in China . The overall response rates were 78.8% (41/52) for PF plus H101, 39.8% (21/53) for PF alone, 50.0% (7/14) for AF plus H101, and 50.0% (2/4) for AF alone, indicating that H101 intratumoral injection showed a distinct antitumor efficacy with acceptable adverse events. This result contributed considerably to the approval of H101 in China as a commercial drug for cancer patients, making H101 the first oncolytic adenovirus to be approved for human use.
Gemcitabine is a nucleoside analog that arrests tumor growth and induces apoptosis by replacing one of the building blocks of nucleic acids, in this case cytidine. Gemcitabine is a relatively novel chemotherapeutic agent that received its first FDA-approved indication in 1996. It is currently used for patients with pancreatic cancer, non-small-cell lung cancer, esophageal cancer, bladder cancer, and breast cancer. A couple of oncolytic adenoviruses in combination with gemcitabine have been tested in preclinical and clinical studies. After a phase I clinical trial of computed tomography-guided intratumoral injection of ONYX-015 into pancreatic cancers, which showed its feasibility and the need for an improved administration method, a phase I/II clinical trial of intratumoral injection of ONYX-015 combined with gemcitabine for 21 patients with pancreatic cancers was performed by endoscopic ultrasound-guided injection via the transgastric route . Partial regressions of greater than 50% occurred in 2 of 21 patients (10%) after combined therapy of ONYX-015 and gemcitabine. Eight patients (38%) had stable disease, and 11 patients (52%) had progressive disease. The combination of Ad5/3-D24, a serotype 3 receptor-targeted oncolytic adenovirus containing a 24-bp deletion in the E1A gene, with gemcitabine for the treatment of ovarian cancer was preclinically examined, and almost 60% of mice treated with Ad5/3-D24 and gemcitabine were cured without obvious side effects in an orthotopic murine model of peritoneally disseminated ovarian cancer . Moreover, a preclinical study of intratumoral injection of Telomelysin, a telomerase-dependent oncolytic adenovirus, in combination with gemcitabine for human lung cancer suggested that this combination had enhanced antitumor effects through the synergistic mechanism of Telomelysin-mediated cell cycle accumulation in S phase, in which gemcitabine can effectively produce antitumor activity .
Alkylating Agents
Cisplatin is a representative chemotherapeutic agent that is currently used for various types of cancers. Cisplatin is the first member of a class of platinum-containing anticancer drugs, which now also includes carboplatin and oxaliplatin. These platinum complexes cross-link DNA in several different ways that interfere with cell division and induce apoptosis if DNA repair is recognized as impossible. Additive or synergistic efficacy of ONYX-015 in combination with cisplatin-based chemotherapy has been shown in in vitro and in vivo studies . ONYX-015 showed the potential to enhance the antitumor effect of cisplatin both in p53-deficient and in p53 wild-type tumor cells. The adenoviral E1A gene product may be involved in this sensitization of p53 wild-type tumor cells. As mentioned previously, a phase II clinical trial of intratumoral ONYX-015 injection combined with cisplatin and 5-FU chemotherapy in patients with recurrent squamous cell cancer of the head and neck was conducted in the United States, and then a phase III trial of this combination was conducted in China, leading to the approval of H101 for human use in China. Interestingly, H101 reportedly sensitized cisplatin-resistant A549, a non-small-cell lung cancer cell line, to cisplatin by downregulating the expression of glutathione- S -transferase and topoisomerase II and increasing the intracellular accumulation of cisplatin . A phase I/II trial of ONYX-015 intratumoral injection in combination with MAP (mitomycin-C, doxorubicin, and cisplatin) in six patients with advanced sarcoma demonstrated that this combination was safe and had evidence of antitumor activity . The intraperitoneal administration of Telomelysin sensitized ovarian cancer cells to cisplatin and prolonged survival in a xenograft mouse model .
Cyclophosphamide is a nitrogen mustard alkylating agent that is used as a chemotherapeutic agent to treat various types of cancer and as an immunosuppressive agent to treat some autoimmune diseases. In terms of the combination of oncolytic adenovirus with cyclophosphamide, cyclophosphamide is supposed to enhance adenoviral infectivity and replication by suppressing the immune response. Intratumoral and intravenous administration of Ad5/3-D24-GMCSF, a 5/3 chimeric oncolytic adenovirus armed with human GM-CSF, combined with low-dose metronomic cyclophosphamide was clinically examined in 21 patients with advanced solid tumors refractory to standard treatments. The combination treatment showed signs of antitumor activity in 13 of the 21 patients (62%) with no severe adverse events .
Mitomycin C, a member of the mitomycin family of aziridine-containing natural products isolated from Streptomyces caespitosus , is a stable chemotherapeutic agent used for a variety of cancers . A phase I clinical trial combining intratumoral injection of ONYX-015 with systemically administered mitomycin C, doxorubicin, and cisplatin in patients with metastatic sarcoma was undertaken to define the maximum tolerated dose of ONYX-015 and assess the antitumor activity of the regimen .
Taxanes
Taxanes are chemotherapeutic agents including paclitaxel and docetaxel that produce antitumor activity by causing stabilization of cellular microtubules, thereby inhibiting cell division . No clinical trial of the combined therapy of oncolytic adenovirus and taxane have been performed, although preclinical studies have shown some potential for this combination therapy. Intravenous administration of CG7870 (also known as CV787), a PSA-positive prostate cell-specific oncolytic adenovirus, in combination with docetaxel demonstrated strong synergistic antitumor efficacy in a prostate cancer xenograft mouse model . The combination of a urothelium-specific oncolytic adenovirus with docetaxel showed synergistic antitumor efficacy in a bladder cancer xenograft mouse model , and the combination of an oncolytic adenovirus expressing tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) with paclitaxel also showed a synergistic effect in a gastric cancer xenograft mouse model . We also demonstrated that Telomelysin that expresses the green fluorescent protein reporter gene under the control of the cytomegalovirus promoter in the E3 region (Telomescan, OBP-401) in combination with docetaxel produced profound antitumor effects against various types of human cancer cell lines in vitro and in vivo . Then, the combined therapy of Telomelysin and paclitaxel showed a greater antitumor effect than either agent alone in a head and neck squamous cell carcinoma xenograft mouse model .
Topoisomerase Inhibitors
Topoisomerase inhibitors are chemotherapeutic agents that interfere with the topoisomerase enzymes (topoisomerase I and II), which control changes in DNA structure . Topoisomerase inhibitors block the ligation step of the cell cycle, which generates DNA single- and double-strand breaks, leading to apoptotic cell death. Topoisomerase I inhibitors include irinotecan, topotecan, and camptothecin, and topoisomerase II inhibitors include etoposide, doxorubicin, and epirubicin. A pilot clinical study of intravenously injected ONYX-015 combined with irinotecan in patients with advanced cancers showed the safety of this combined therapy . As underlying preclinical data, an in vitro study showed that the combination of ONYX-015 and irinotecan increased the induction of apoptosis in human colon cancer cell lines regardless of p53 status . In a study to determine if Ad5-D24, an oncolytic adenovirus that has a 24-bp deletion in the E1A gene, could sensitize glioma cells to irinotecan in vitro and in vivo , Ad5-D24 enhanced the effect of irinotecan by accumulating cells in the S phase, in which irinotecan is most effective, and the combination of these agents resulted in significantly prolonged animal survival in a glioma xenograft mouse model .
As mentioned previously, a phase I/II clinical trial of ONYX-015 in combination with MAP chemotherapy in patients with advanced sarcomas showed the safety of this combined therapy and provided evidence of antitumor activity in one of the six patients . ONYX-015 demonstrated synergistic effects with doxorubicin in an in vitro study of human anaplastic thyroid carcinoma cell lines . The combination of CV890, a hepatocellular carcinoma (HCC)-specific oncolytic adenovirus, with doxorubicin showed synergistic antitumor effects, eradicating HCC xenografts in mice after a single intravenous administration of both agents . In another report, the intraperitoneal administration of Ad5/3-D24 in combination with epirubicin yielded strong antitumor activity and increased the survival of mice in an orthotopic ovarian cancer model .
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree