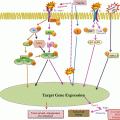
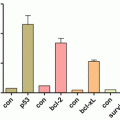
Fig. 23.1
Inhibition of tumorsphere formation by CQ
Conclusions
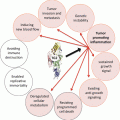
Since small subpopulations of CSCs were identified from blood mononuclear cells in human acute myeloid leukemia in 1997, it has attracted a great deal of attention recently benefited with advances in SC biology as well as the revisited concept of cell quiescence or tumor dormancy. Also, as the reason why a small population of cancer cells referred to CSCs have received particular attention, CSCs were responsible for acquiring stem cell-like properties and becoming the main cause of tumor propagation and metastasis as well as chemotherapeutic or radiation resistance. Though many CSC-targeted therapy methods were expected to cure or prevent cancer by eradicating CSCs, it has not come true clinically yet. Since the identification of CSC-specific markers, the isolation and characterization of CSCs from malignant tissues, and targeting strategies for the destruction of CSCs might provide a novel opportunity for cancer research, huge efforts are now on progress. Repositioning research for metformin, CQ, PPI, APA, and Shh inhibitor might provide unexpected discovery for chemoquiescence in the near future, the expected translational impact of the “old drugs-new uses” repurposing strategy to open a new CSC-targeted chemoprevention era (Fig. 23.2) (Vazquez-Martin et al. 2011; Del Barco et al. 2011). Ongoing chemopreventive, neoadjuvant, and adjuvant trials should definitely establish whether metformin’s ability to kill the “dandelion root” beneath the “cancer soil” likely exceeds metformin-related dangers of hormesis (Del Barco et al. 2011). From our investigation as shown in Fig. 23.1, CQ can be one of the most effective and safe sensitizers for cancer therapies based on results of our and other investigations focused on the changes of tumorsphere after CQ. Taken together, the efficacy of conventional cancer therapies can be dramatically enhanced with the combination of CQ and its analogs (Kimura et al. 2013; Solomon and Lee 2009; Maycotte et al. 2012), with the possibility of achievement of chemoquiescence in the near future after extensive clinical investigation.
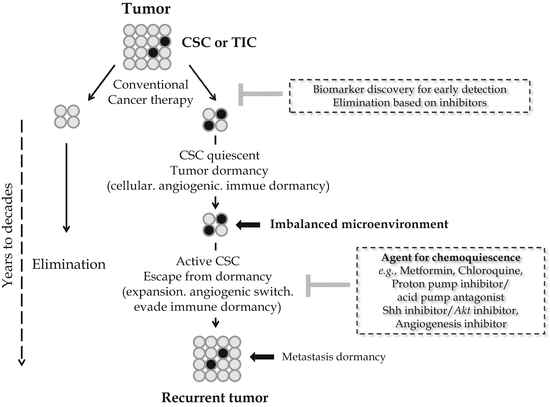

Fig. 23.2
Chemoquiescence for preventing cancer progression and recurrence
References
Ailles LE, Weissman IL (2007) Cancer stem cells in solid tumors. Curr Opin Biotechnol 18(5):460–466. doi:10.1016/j.copbio.2007.10.007 PubMedCrossRef
Al-Hajj M, Clarke MF (2004) Self-renewal and solid tumor stem cells. Oncogene 23(43):7274–7282. doi:10.1038/sj.onc.1207947 PubMedCrossRef
Alison MR, Lin WR, Lim SM, Nicholson LJ (2012) Cancer stem cells: in the line of fire. Cancer Treat Rev 38(6):589–598. doi:10.1016/j.ctrv.2012.03.003 PubMedCrossRef
Almog N (2010) Molecular mechanisms underlying tumor dormancy. Cancer Lett 294(2):139–146. doi:10.1016/j.canlet.2010.03.004 PubMedCrossRef
Balic A, Draeby Sorensen M, Trabulo SM, Sainz B Jr, Cioffi M, Vieira CR, Miranda-Lorenzo I, Hidalgo M, Kleeff J, Erkan M, Heeschen C (2014) Chloroquine targets pancreatic cancer stem cells via inhibition of CXCR4 and hedgehog signaling. Mol Cancer Ther. doi:10.1158/1535-7163.MCT-13-0948 PubMed
Borst P (2012) Cancer drug pan-resistance: pumps, cancer stem cells, quiescence, epithelial to mesenchymal transition, blocked cell death pathways, persisters or what? Open Biol 2(5):120066. doi:10.1098/rsob.120066 PubMedCentralPubMedCrossRef
Buczacki S, Davies RJ, Winton DJ (2011) Stem cells, quiescence and rectal carcinoma: an unexplored relationship and potential therapeutic target. Br J Cancer 105(9):1253–1259. doi:10.1038/bjc.2011.362 PubMedCentralPubMedCrossRef
Campos B, Gal Z, Baader A, Schneider T, Sliwinski C, Gassel K, Bageritz J, Grabe N, von Deimling A, Beckhove P, Mogler C, Goidts V, Unterberg A, Eckstein V, Herold-Mende C (2014) Aberrant self-renewal and quiescence contribute to the aggressiveness of glioblastoma. J Pathol. doi:10.1002/path.4366 PubMed
Chen K, Huang YH, Chen JL (2013) Understanding and targeting cancer stem cells: therapeutic implications and challenges. Acta Pharmacol Sin 34(6):732–740. doi:10.1038/aps.2013.27 PubMedCentralPubMedCrossRef
Cheng L, Alexander R, Zhang S, Pan CX, MacLennan GT, Lopez-Beltran A, Montironi R (2011) The clinical and therapeutic implications of cancer stem cell biology. Expert Rev Anticancer Ther 11(7):1131–1143. doi:10.1586/era.11.82 PubMedCrossRef
Cheung TH, Rando TA (2013) Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol 14(6):329–340. doi:10.1038/nrm3591 PubMedCrossRef
Croker AK, Allan AL (2008) Cancer stem cells: implications for the progression and treatment of metastatic disease. J Cell Mol Med 12(2):374–390. doi:10.1111/j.1582-4934.2007.00211.x PubMedCrossRef
Del Barco S, Vazquez-Martin A, Cufi S, Oliveras-Ferraros C, Bosch-Barrera J, Joven J, Martin-Castillo B, Menendez JA (2011) Metformin: multi-faceted protection against cancer. Oncotarget 2(12):896–917PubMedCentralPubMed
Firat E, Weyerbrock A, Gaedicke S, Grosu AL, Niedermann G (2012) Chloroquine or chloroquine-PI3K/Akt pathway inhibitor combinations strongly promote gamma-irradiation-induced cell death in primary stem-like glioma cells. PLoS One 7(10):e47357. doi:10.1371/journal.pone.0047357 PubMedCentralPubMedCrossRef
Giancotti FG (2013) Mechanisms governing metastatic dormancy and reactivation. Cell 155(4):750–764. doi:10.1016/j.cell.2013.10.029 PubMedCrossRef
Hadnagy A, Gaboury L, Beaulieu R, Balicki D (2006) SP analysis may be used to identify cancer stem cell populations. Exp Cell Res 312(19):3701–3710. doi:10.1016/j.yexcr.2006.08.030 PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree