9 Screening of High-Risk Patients
Definition of High Risk
Identification of risk factors and stratification of patients for breast cancer risk are important for providing the best screening recommendations. Many factors that increase or decrease risk have been identified, and several statistical models, such as the Gail model and the Claus model, calculate risk based on these factors. The Gail model is the most commonly used methodology for calculating risk factors; however, some experts believe that the BRACAPRO or Tyrer-Cuzick models provide more accurate assessments of risk.1 “High risk” has been arbitrarily defined as a 5-year Gail model risk of greater than 1.7% and a lifetime risk based primarily on family history of greater than 20%. Other situations or conditions that place a woman at high risk for breast cancer development are presented in Table 9-1. The National Cancer Institute and the National Surgical Adjuvant Breast and Bowel Project (NSABP) worked together to provide a free calculator based on the Gail model, which assesses some of these risk factors and provides an estimated 5-year and lifetime risk rate for a woman’s developing breast cancer compared with the “average woman” (www.cancer.gov/bcrisktool/). The patient and her breast care provider should discuss the best screening plan based on the individual woman’s estimated risk.
Table 9-1 Recommendations for Breast MRI Screening as an Adjunct to Mammography
| Insufficient Evidence to Recommend for or Against MRI Screening‡ |
Lifetime risk 15–20%, as defined by BRCAPRO or other models that are largely dependent on family history |
* Evidence from nonrandomized screening trials and observational studies.
† Based on evidence of lifetime risk for breast cancer.
‡ Payment should not be a barrier. Screening decisions should be made on a case-by-case basis, since there may be particular factors to support MRI. More data on these groups are expected to be published soon.
From Warner E, Yaffe M, Andrews KS, et al: American Cancer Society Guidelines for Breast Screening with MRI as an Adjunct to Mammography. CA Cancer J Clin 57:75–89, 2007, Table 1.
Mammography
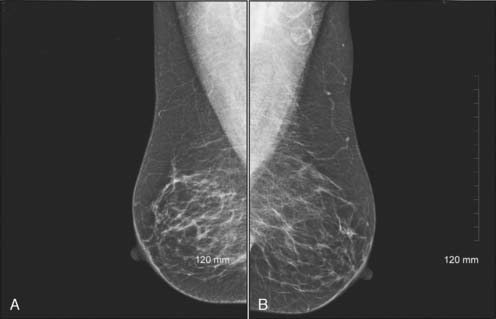
Mammography, or breast x-ray, is the primary imaging modality used to screen women for occult breast cancer (Fig. 9-1). A standard screening mammogram involves two images of each breast (Figs. 9-2A and B and 9-3A and B). Studies have shown that two views increase the rate of detection of small cancers (more favorable prognosis) and decrease the number of patients being called back for additional images.2–4 The benefits just mentioned outweigh the minimal risks of additional radiation exposure received with only one view. The two images are named on the basis of the orientation of the view: MLO for mediolateral oblique (see Fig. 9-2) and CC for craniocaudal (Fig. 9-3). It is interesting that the effective whole body radiation dose from a screening mammogram is 40 mrem, which is equal to 10% of the annual background radiation and is within the range of normal background variation.

Figure 9-1 Digital mammography system.
(Courtesy of Brian M. Bagrosky MD, Dianne O’Connor Thompson Breast Center, University of Colorado Hospital, Aurora, CO.)
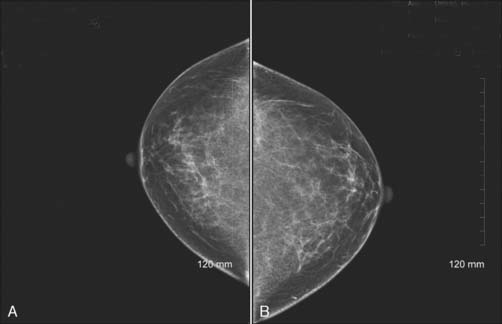
Figure 9-3 Bilateral digital screening mammogram: craniocaudal views of the left (A) and right (B) breasts of the same patient as in Figure 9-2.
(Courtesy of Brian M. Bagrosky MD, Dianne O’Connor Thompson Breast Center, University of Colorado Hospital, Aurora, CO.)
Many technologic advances to mammography units have dramatically improved the image quality and given radiologists a better ability to identify very small distortions in normal breast architecture, small masses, and microcalcifications.5 Two of the most important advances have been compression paddles and the replacement of tungsten filament with molybdenum and rhodium. Although compression paddles (Fig. 9-4) can cause discomfort in some patients, they serve to spread the breast tissue out, creating less tissue thickness for the x-ray beam to penetrate and therefore decreasing scatter. Compression paddles also decrease motion during the imaging, which markedly improves the final images. Replacing tungsten filaments and aluminum filters (found in conventional x-ray units) with molybdenum and/or rhodium filaments and filters creates “softer” x-rays, which provide better imaging of very small differences in breast soft tissue attenuation. In addition to technologic advances in mammography units, the Mammography Quality Standards Act (MQSA) written by the Food & Drug Administration (FDA) and passed by Congress in October 1992 has created strict guidelines that all breast imaging centers must adhere to for accreditation. These guidelines include daily film processor testing, weekly radiation dose testing, monthly image quality testing, and annual physics testing. The MQSA also sets a guideline on the minimum number of studies a radiologist must read every 2 years to be accredited. The MQSA guidelines have improved the specificity and sensitivity of all mammograms by an estimated 2% to 10%.6
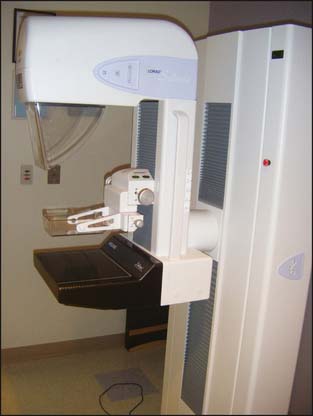
Figure 9-4 Compression paddle in mammography unit.
(Courtesy of Brian M. Bagrosky MD, Dianne O’Connor Thompson Breast Center, University of Colorado Hospital, Aurora, CO.)
Despite the technologic advances and the rigorous quality standards, mammography has skeptics. The National Breast Cancer Coalition (NBCC) is a grassroots lobbying group with a stated mission of eradicating breast cancer. This coalition takes a guarded stance on mammography, stating: “The National Breast Cancer Coalition Fund (NBCCF) believes, on the basis of recently published reviews, that the benefits of screening mammography in reducing mortality are modest and there are harms associated with screening.”7 The NBCC cites the Cochrane review database, a respected meta-analysis, which has analyzed seven published studies on mammography screening for breast cancer. The conclusion of the Cochrane review in 2001 was that mass screening for breast cancer by mammography did not show a survival benefit. However, the later Cochrane review in 2006 concluded that mammography likely decreases breast cancer mortality, but that some women who partake in screening will undergo unnecessary further imaging and treatment because of false-positive results. This is a true and unfortunate part of any screening program for any disease.8 Currently, it is estimated that mammography and early treatment of breast cancer are responsible for lowering breast cancer death rates by as much as 50% in the last 2 decades.9
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree