(1)
Department of Clinical Cancer Prevention, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Chapter Overview
Significant improvements in early detection and advances in cancer treatment in the past few decades have resulted in increasing numbers of cancer survivors. Given the major improvements in survival rates and durations, identification and characterization of the late sequelae of cancer and its treatment have become critical.
It is well known that cancer survivors are at risk for recurrence of the primary cancer. The risk of developing a second primary cancer (SPC) is also increased. In view of the increasing number of cancer survivors, the development of SPCs has emerged as a significant problem that can affect quality of life and long-term survival. In addition to recurrence of the primary cancer, the diagnosis of a new cancer represents one of the most serious events experienced by cancer survivors.
Interest in this area has increased owing to the potential for reducing the risk for SPCs through an understanding of genetic predispositions; lifestyle, behavioral, and environmental factors; and treatment-related effects that influence the development of SPCs. An understanding of the risks for SPCs can guide risk reduction strategies and cancer screening recommendations, with the goal of preventing SPCs or providing early detection and intervention.
Introduction
Survival from cancer has improved dramatically as a result of the early detection of cancer and advances in cancer treatment. As of 2007, about 12 million people in the United States were living with cancer. As the number of cancer survivors, as well as the duration of cancer survivorship, has increased, the incidence of second primary cancers (SPCs) has also increased. Accordingly, 1 in 6 cancer incidents (16%) reported to the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program is a SPC, making SPCs an important area of concern for patients and their physicians (Ries et al. 2006). Cancer survivors have approximately twice the probability of developing a new primary cancer compared with cancer-free individuals of the same age and risk (Krueger et al. 2008). In fact, excluding non-melanoma skin cancer, SPCs are now the fifth most common category of malignancy, behind lung, colorectal, breast, and prostate cancer (Rheingold et al. 2000).
It is important to recognize that SPCs are a by-product of medical success. If patients do not survive the primary cancer, they are not at risk for a SPC. In some patients, the prognosis is so poor with the primary cancer that there is little opportunity for the development of a SPC. As treatments improve, our understanding of the incidence of SPCs in patients with primary cancers that have a poor prognosis will naturally increase.
Definition of Second Primary Cancer
A seemingly straightforward definition of SPC is a new primary cancer in a person with a history of cancer. However, upon consideration, this definition is significantly more simplistic than originally realized, and it raises a number of questions. For example, are contralateral tumors in paired organs (e.g., breast, kidney) considered SPCs? What about second tumors within the same field (e.g., head and neck, colon)?
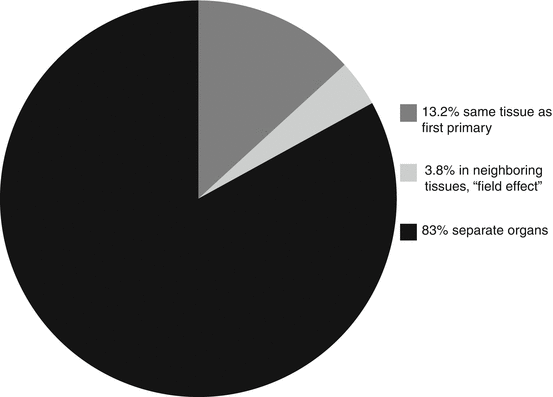
New malignancies that occur in the same site or organ as the primary cancer have been shown to account for 13.2% of the SPCs occurring among patients surviving 2 months or more; new tumors in the female breast (7.2%), colon (2%), and lung (1.8%) and melanoma of the skin (0.9%) made up most of these cases (Fraumeni et al. 2006). An additional 3.8% of SPCs originated in neighboring tissues or organs (e.g., within the head and neck, colorectum, or lower urinary tract), a result of a “field cancerization” process whereby carcinogenic exposures and susceptibility states contribute to multicentric tumors (Fraumeni et al. 2006). Molecular studies of some of these cancers indicated that multicentric involvement may actually result from the spread and implantation of a single clone of mutated cells and may involve effects of carcinogenic exposures or genetic factors over areas of tissue or organs. The oral cavity and pharynx site seems to be associated with the highest risk of multicentric tumors; ratios of observed SPCs in survivors to expected cancers in the general population (O/E ratios) at this site were 29.5 in women and 11.5 in men (Fraumeni et al. 2006).
However, 83% of SPCs reported in the SEER database arose in separate or independent organ systems (Fraumeni et al. 2006; Fig. 18.1). This constellation of cancers presents unique opportunities for primary prevention and may require the implementation of earlier or additional cancer screenings in cancer survivors to ensure early detection of disease outside of the primary cancer site. It is not uncommon for monitoring in cancer survivors to be focused on the site of the first primary cancer. The intent of this chapter is to identify cancer risks beyond the site of the first primary cancer. These risks may be related to the first primary cancer or independent of it, based solely on the aging of the survivor population. These risks present a unique opportunity to counsel survivors regarding risk reduction and screening strategies.
For the purposes of this chapter, the term SPCs refers to neoplasms that arise independently in a new site or tissue at least 2 months after the primary cancer is diagnosed (Krueger et al. 2008).
Incidence Patterns
The risks for SPCs vary by site of primary cancer, age at diagnosis, sex, and race. Analysis of large cancer registries has consistently revealed that the overall O/E ratio for SPCs is approximately 2.0 (Krueger et al. 2008). In some patient populations, research has demonstrated no increased risk for SPCs at all (e.g., among patients with prostate cancer). However, dramatically increased risks for SPCs have been reported in other patient populations (the O/E ratio is ~16 for tongue cancer after laryngeal cancer, ~34 for cancer of the small intestine after colorectal cancer, and ~14 for vaginal cancer after cervical cancer; Krueger et al. 2008). It is important to identify specific populations that are at increased risk for SPCs and require more intensive risk management and screening for SPCs.
Striking differences in the incidence of SPCs have been observed by age at diagnosis of the primary cancer; the risk for SPCs among survivors of childhood cancer (i.e., those diagnosed with a primary cancer between the ages of 0 and 17 years) is more than six times higher than in the general population (O/E = 6.13; Table 18.1). An age effect is further illustrated by the 2- to 3-fold increased risks for SPCs among patients diagnosed with the primary cancer as young adults (ages 18–39 years) compared with the 1.2- to 1.6-fold increased risks among those diagnosed at ages 40–59 years. In contrast, the observed number of SPCs is lower than expected among survivors whose primary cancer was diagnosed at age ≥80 years (O/E = 0.92). This is likely due to competing risks from comorbid conditions and shortened life expectancy in this population (Fraumeni et al. 2006).
Table 18.1
Risk of developing a second primary cancer by age at diagnosis of primary cancer (n = 2,036,597)
Age at diagnosis of primary cancer | O/E ratioa |
|---|---|
All ages | 1.14 |
0–17 years | 6.13 |
18–29 years | 2.92 |
30–39 years | 2.37 |
40–49 years | 1.61 |
50–59 years | 1.27 |
60–69 years | 1.13 |
70–79 years | 1.02 |
80–115 years | 0.92 |
Overall, women have a slightly higher risk of developing SPCs than men (O/E = 1.17 vs 1.11). This difference is likely due to the increased risk of developing a SPC in the breast and gynecologic organs among women, as well as the longer life expectancy of female cancer survivors (Krueger et al. 2008; Altekruse et al. 2010). However, the risk for SPCs in men consistently exceeded the risk for SPCs in women among patients whose primary cancer was diagnosed before the age of 60 years.
For all ages combined, black cancer survivors had a higher risk of developing a SPC than white cancer survivors (O/E = 1.13; Fraumeni et al. 2006).
Mechanisms
Although a certain fraction of SPCs in cancer survivors would be expected to develop at the same rate as primary cancers in the general population, the patterns of increased risk in cancer survivors are sufficiently distinctive to suggest that primary cancers and SPCs may share the same risk factors, or that cancer therapies may have potentially carcinogenic effects. Insights into carcinogenic pathways of SPCs can guide the approach to cancer screening and prevention among cancer survivors to reduce their risk for SPCs.
Three carcinogenic pathways have been outlined for SPCs (Krueger et al. 2008; Table 18.2):
Table 18.2
Carcinogenic pathways of second primary cancers
Carcinogenic pathway | Examples |
|---|---|
Genetic predisposition | Lynch syndrome, BRCA, Li Fraumeni syndrome, Cowden syndrome, and others |
Lifestyle, behavioral, or environmental factors | Tobacco or alcohol consumption, obesity (poor diet and lack of exercise), sun exposure, infections (e.g., human papillomavirus, hepatitis B and C, human immunodeficiency virus, Helicobactor pylori) |
Treatment-related effects | Radiation therapy, chemotherapy, hormonal therapy (e.g., tamoxifen) |
1.




Genetic factors involved in both the primary cancer and the SPC.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree