Yves Rolland, Matteo Cesari, Bruno Vellas
Sarcopenia
In a paper published in 1997,1 Irwin Rosenberg described how the term sarcopenia (from Greek, sarx “flesh” and penia “loss”) was coined:
In 1988, we convened a meeting in Albuquerque, New Mexico to look at various measurements related to the assessment of health and nutrition in elderly populations. … I noted that no decline with age is as dramatic or potentially more significant than the decline in lean body mass. In fact, there may be no single feature of age-related decline more striking than the decline in lean body mass in affecting ambulation, mobility, energy intake, overall nutrient intake and status, independence and breathing. I speculated as to why we had not given this more attention and suggested … we had to give it a name. This would provide recognition by the scientific community and by the National Institutes of Health. … I proposed … sarcomalacia or sarcopenia.
Since then, the understanding of the aging process has improved, and better and more widely diffused techniques are available for measuring body composition. Nevertheless, the clinical implementation of sarcopenia is still delayed by multiple problems affecting its clear framing and operationalization.2 To date, sarcopenia is still not recognized as a nosologic entity.
Operational Definitions
Skeletal muscle decline has been operationalized in a number of ways over the years, especially to translate the theoretical concept of the age-related skeletal muscle decline into a clinically meaningful condition of older age. A major challenge has been generating a diagnostic algorithm capable of capturing the two domains of the sarcopenia condition—skeletal muscle mass loss (i.e., a quantitative parameter) and skeletal muscle function loss (i.e., a qualitative parameter). This bidimensional nature of the definition implies the simultaneous assessment as well as the parallel and equivalent consideration of the two factors for the detection of sarcopenia.
The first operational definition of sarcopenia was the one provided by Baumgartner and colleagues3 in a study of participants recruited in the New Mexico Elder Health Survey and a reference group of young individuals. Sarcopenia was defined as the presence of an appendicular lean mass (weight/height2) below 7.26 kg/m2 in men and 5.45 kg/m2 in women. Subsequently, it was pointed out that the predictive value of the skeletal muscle mass alone for negative outcomes in older adults was not particularly striking.4,5 At the same time, some studies demonstrated that the best prediction for negative health-related end points was reached when muscle mass was simultaneously considered together with fat mass. For example, Newman and and coworkers,6 in the Health Aging and Body Composition Study, even proposed a different model of operational definition for sarcopenia based on fat-adjusted appendicular lean mass residuals.
All the early operational definitions available in the sarcopenia literature were particularly focused on quantification of the skeletal muscle mass. Such an approach used the characteristics of a research condition rather than a clinical entity in relation to the condition studied. To more closely approximate the sarcopenia condition to clinical practice, especially for adopting it as a target of interventions against mobility disability), several panels of experts provided operational definitions, including the second (qualitative) domain of the skeletal muscle decline—that is, the muscle dysfunction. Thus, at least four different consensus papers (Table 72-1) were released in a few years proposing to define sarcopenia on the basis of algorithms, simultaneously considering a quantitative estimation of the muscle mass and qualitative estimation of muscle performance.7–11 Unfortunately, although conceptually similar, the four definitions had key differences. For example, the instrument for quantifying the muscle mass was variable, when specifically indicated. Also, different measures of physical function and muscle strength, with different thresholds of risk, were proposed. Furthermore, the algorithms used to define the sarcopenic individual differently considered the measured components.
TABLE 72-1
Main Operational Definitions of Sarcopenia*
| Parameter | International Working Group on Sarcopenia7 | European Working Group on Sarcopenia in Older People9 | ESPEN Special Interest Group on Cachexia-Anorexia in Chronic Wasting Diseases10 | Society of Sarcopenia, Cachexia and Wasting Disorders8 |
| Target population | Subjects with clinical declines in physical function, strength, or health status | All persons ≥ 65 yr | Older adults | Persons > 60 yr with clinical declines in physical function, strength, or health status; exclusion of specific muscle diseases, peripheral vascular disease with intermittent claudication, central and peripheral nervous system disorders, and cachexia |
| Screening | Physical function (4-m usual GS test); if GS < 1.0 m/sec, proceed to BC evaluation | If GS ≤ 0.8 m/sec, proceed to BC evaluation; if GS > 0.8 m/sec, measure hand grip strength; if muscle weakness present, proceed to BC evaluation. | Distance walked during 6-min walk test (cut point, 400 m), or GS < 1.0 m/sec (4- to 6-m track length) | |
| Operative definition | Poor functioning plus poor ALM (assessment by DXA)3 | Low muscle mass in subjects with GS ≤ 0.8 m/sec or normal GS but low muscle strength | Low muscle mass (≥2 SDs below mean measured in young adults of same gender and ethnic group) plus slow GS (<0.8 m/sec on 4-m track); GS can be replaced by another physical performance measure | Poor functioning plus low ALM (≥2 SDs below mean measured in healthy persons aged 20-30 yr from same ethnic group) |
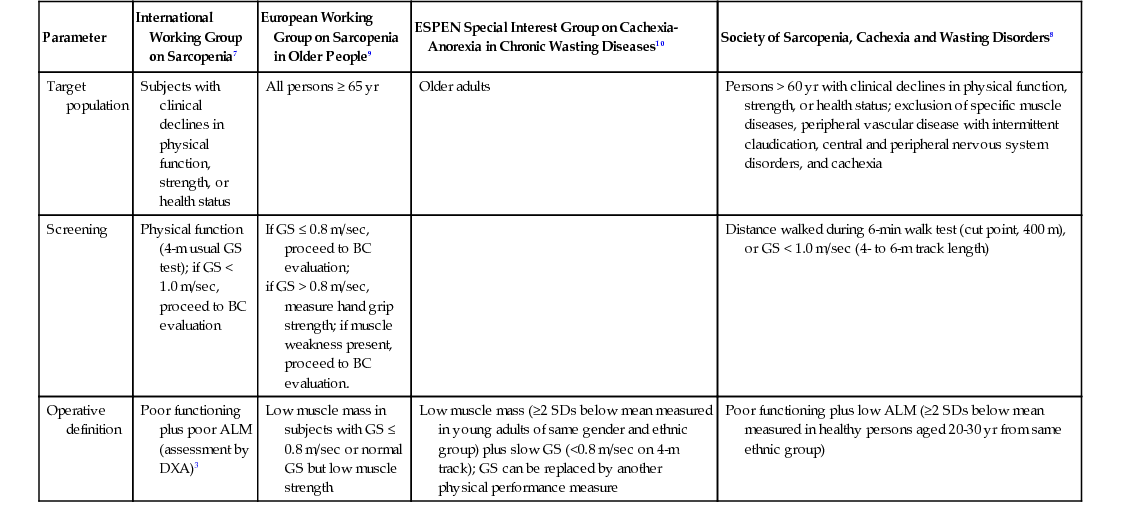
Another problem was present from the very beginning. Muscle mass and muscle function have different clinical relevance.4,5,12 From a geriatric viewpoint, the size of the organ is not particularly relevant, but rather its capacity to sustain the individual at maintaining his or her independent life. Moreover, the age-related declines of muscle mass and muscle function do not follow similar trajectories.13 The former seems to be relatively more stable over time compared to muscle strength. This might explain why muscle function can discriminate different risk profiles in older adults better. In this context, it is also noteworthy that the combination of quantitative and qualitative parameters in the same algorithm has always been operationalized in an additive way; that is, the two dimensions of sarcopenia are of equal weight in determination of the phenotype and clinical relevance. However, this has been disproved by the growing number of studies demonstrating that the predictive value of sarcopenia definitions for negative health-related events is largely driven by the skeletal muscle quality (physical performance measures) rather than its quantity (skeletal muscle mass).4,5,12 It is also noteworthy that the clinical relevance of sarcopenia-related parameters is also highly influenced by gender,12 so that muscle strength parameters seem more predictive of negative outcomes in men, whereas body composition variables, especially those considering adipose tissue, are stronger predictors in women.
With the objective of bypassing all the issues related to the definition of sarcopenia, the Foundation of National Institutes of Health (FNIH) Sarcopenia Project has recently released results from longitudinal observational analyses conducted in a large sample of participants in several cohort studies worldwide.14 Investigators decided to adopt a different approach compared to what had been proposed in the available consensus definitions. Slow gait speed, which had been previously used as a component of the sarcopenia definitions, became the outcome of models that defined gender-specific optimal cut points for the most predictive parameters of body composition and, separately, muscle strength (Table 72-2).
TABLE 72-2
Definition of Low Muscle Mass and Poor Muscle Function*
| Parameter | Men | Women |
| MUSCLE WEAKNESS | ||
| Hand grip strength (recommended) | <26 kg | <16 kg |
| BMI-adjusted hand grip strength (alternate) | <1.0 kg | <0.56 kg |
| LOW MUSCLE MASS | ||
| BMI-adjusted ALM (recommended) | <0.789 | <0.512 |
| ALM (alternate) | <19.75 kg | <15.02 kg |

Epidemiology
After 50 years of age, muscle mass is reported to decline at an annual rate of approximately 1% to 2%, but strength declines at 1.5%/year and accelerates to as much as 3%/year after the age of 60 years.15 These rates are high in sedentary individuals and twice as high in men as compared to women.16 However, men, on average, have larger amounts of muscle mass and shorter survival than women, which implies that sarcopenia is potentially a greater health concern in women than in men.
It is difficult to estimate the prevalence of sarcopenia in older adults accurately and definitively due to the multiple ambiguities and controversies existing in its operational definition. Moreover, the need to quantify muscle mass limits the large-scale assessment of the phenomenon. In the New Mexico Elder Health Survey, sarcopenia was found to affect about 20% of men between the ages of 70 and 75 years and about 50% of those older than 80 years; 25% to 40% of women have sarcopenia in the same age ranges.3 However, Baumgartner later recognized that these estimates, based on a bioelectric impedance equation, might be biased and published revised prevalence estimates based on dual-energy x-ray absorptiometry (DXA) studies; these ranged from 8.8% in women and 13.5% of men aged 60 to 69 years and up to 16% in women and 29% in men older than 80 years.17 In a healthy, older, community-dwelling population 70 years of age and older in the French Epidemiologie de l’Osteoporose (EPIDOS) study,18 only 10% of women had sarcopenia based on the Baumgartner’s index, but cut-points derived from a different reference group. Using a similar definition, Janssen and coworkers reported retrospectively that 35% of older adults in the population-based National Health and Nutritional Examination Survey III (NHANES III) had a moderate degree of sarcopenia and 10% had a severe degree of sarcopenia.19 Findings from a separate study by Melton and colleagues using yet another definition has suggested that sarcopenia affects 6% to 15% of persons older than 65 years.20
In a recent report,21 the prevalence of sarcopenia defined according to the European Working Group on Sarcopenia in Older People (EWGSOP) algorithm was found to be influenced by regional and age-related variations. However, the authors estimated its prevalence to be 1% to 29% in community-dwelling populations, 14% to 33% in long-term care populations, and 10% in those in acute hospital care units.
Causes
Multiple risk factors and mechanisms contribute to the development of sarcopenia. Lifestyle behaviors such as physical inactivity, poor diet, and age-related changes in hormones and cytokine levels are important risk factors. Postulated mechanisms include alterations in muscle protein turnover, muscle tissue remodeling, loss of alpha motor neurons, and muscle cell recruitment and apoptosis.22,23 Genetic susceptibility also plays a role and explains individual and group differences in rates of sarcopenia. Each factor in regard to the cause and pathogenesis of sarcopenia potentially contributes differently to the loss of muscle mass, strength, or quality, although the relative influences of each factor on sarcopenia is not yet well understood.
Lack of Physical Activity
Inactivity is an important contributor to the loss of muscle mass and strength at any age.24–26 Lifelong physical exercise has shown to delay the age-associated skeletal muscle decline.27 Inactivity results from bed rest studies have indicated that a decrease in muscle strength occurs before a decrease in muscle mass.28
Loss of Neuromuscular Function
The neurologic contribution to sarcopenia occurs through a loss of alpha motor neuron axons.29 Decreased electrophysiologic nerve velocity, related to the dropout of the largest fibers, reduces internodal length, and segmental demyelination occurs with the aging process,15 but the role of demyelination in sarcopenia seems minor.30 The central drive that contributes to a decrease in voluntary strength is supposed to be preserved. The progressive denervation and reinnervation process observed during aging that results in fiber type grouping is the potential primary mechanism involved during the development of sarcopenia. From cross-sectional findings, the decline in motor neurons starts after the seventh decade, with a loss of about 50% of alpha motor neurons,31 and this affects the lower extremities, with their longer axons, more than the upper limbs.15 The reduction in alpha motor neuron number and in motor unit numbers results in a decline in coordinated muscle action and a reduction in muscle strength. Reinnervation contributes to the final differentiation of nerve fibers and the repartition between the type I fibers (slow oxidative fibers) and type II fibers (fast glycolytic fibers).
During aging, the number of satellite cells and their recruitment ability decrease, with a greater decrease in type II than type I fibers.32 Satellite cells are myogenic stem cells that can differentiate to new muscle fibers and new satellite cells if activated during the process of regeneration,33 but this regeneration may lead to imbalance, and the number of type II muscle fibers may decline following damage.
Altered Endocrine Function
There is evidence linking age-related hormonal changes to the loss of muscle mass and muscle strength. However, controversy persists regarding their respective roles and effects on skeletal muscle in adulthood and old age.
Insulin
In young adults, muscle protein synthesis is stimulated by insulin, regardless of glucose tolerance.34 This anabolic capacity seems to decrease with aging due to insulin resistance (at least partly due to the progressive increase in body and intramyocellular fat mass20) and mammalian target of rapamycin complex-1 (mTORC1) signaling.35,36 The mTORC1 pathway is important to modulate muscle growth by serving as a modulator sensitive to nutritional, hormonal, and exercise stimuli.37
Estrogens
There have been conflicting data on the effects of estrogens on sarcopenia. Epidemiologic and interventional studies have suggested that estrogens prevents the loss of muscle mass38,39 because their decline with age increase the levels of proinflammatory cytokines suspected to be involved in the sarcopenia process, such as tumor necrosis factor-α (TNF-α) and interleukin (IL)-6.40 Estrogens also increase the level of sex hormone-binding globulin, which reduces the level of serum-free testosterone, so hormone replacement therapy (HRT) should decrease rather than increase muscle mass.41 Both these mechanisms may play a marginal role involving estrogen during the development of sarcopenia.
Growth Hormone and Insulin-Like Growth Factor 1
Circulating levels and the pulsatile release of growth hormone (GH) are usually decreased in older adults. Therefore, it has been hypothesized that GH might be useful in preventing skeletal muscle decline. Insulin-like growth factor 1 (IGF-1) activates satellite cell proliferation and differentiation and increases protein synthesis in existing fibers.42 There is also evidence that IGF-1 acts in muscle tissue by interacting with androgens,43 but there are conflicting results regarding its effect on muscle strength, despite the apparent increase in muscle mass.44
Testosterone
Testosterone levels gradually decrease in older men at a rate of 1%/year, and epidemiologic studies have suggested a relationship between low levels of testosterone in older men and loss of muscle mass, strength, and function. The increase in sex hormone–binding globulin levels with age results in lower levels of free or bioavailable testosterone. Clinical and experimental studies have supported the hypothesis that low testosterone levels predict sarcopenia, with low testosterone levels resulting in lower protein synthesis and a loss of muscle mass.45 Testosterone induces an increase numbers of satellite cells in a dose-dependent manner, which is a major regulating factor of satellite muscle cell function.43 When administered to hypogonadal subjects or older men with low levels, testosterone46 increased muscle mass, muscle strength, and protein synthesis. Despite evidence that dehydroepiandrosterone (DHEA) supplementation results in an increase of blood testosterone levels in women and an increase of IGF-1 levels in men, few studies have reported an effect on muscle size, strength, or function.47
Vitamin D and Parathyroid Hormone
With aging, 25-OH vitamin D levels decline.48 Several studies have reported a close relationship between low 1,25-OH vitamin D levels and low muscle mass, low muscle strength, decreased balance, and increased risk of falls.48 The nuclear 1,25-OH vitamin D receptor has been described in muscle cells,49 and low levels of vitamin D have been shown to decrease muscle anabolism. Low vitamin D levels may also influence muscle protein turnover through reduced insulin secretion. Low levels of vitamin D are associated with raised parathyroid hormone (PTH) levels, but other studies have suggested that a high PTH level is also independently associated with sarcopenia.38,50
Chronic Inflammatory Status
Chronic medical conditions, such as chronic obstructive pulmonary disease (COPD), heart failure, and cancer, are highly prevalent in older adults and are associated with an increased serum level of proinflammatory cytokines and loss of body weight, including lean mass. This condition can occur in younger or older adults is termed cachexia.51 This acute hypercatabolism differs from the long-term, age-related process that leads to sarcopenia. However, aging is also associated with a more gradual, chronic, increased production of proinflammatory cytokines, particularly IL-6 and IL-1, by peripheral blood mononuclear cells. There is some evidence that increased fat mass and reduced circulating levels of sex hormones with aging contribute to this age-related increase in proinflammatory cytokines, which constituted catabolic stimuli.52–54 Thus, the aging process itself is associated with increased catabolic stimuli, and there is evidence for the hypothesis that cytokines (in particular, TNF-α) predict skeletal muscle decline.55
Adipose tissue is an endocrine organ involved in the secretion of proinflammatory cytokines.56 The close relationship existing between adipose tissue and skeletal muscle justifies the development of the worst case scenario known as sarcopenic obesity, in which the excess of fat mass and reduction of lean mass are present simultaneously.57–59 Sarcopenic obesity has been reported to predict the onset of disability more than sarcopenia or obesity alone. As for sarcopenia, a unique and clear estimation of the prevalence of the sarcopenic condition is not possible. Several definitions of sarcopenic obesity have been proposed; each is legitimate but differs from the others for the assessment of sarcopenia and obesity. Overall, data have suggested that women tend to have a higher prevalence of sarcopenic obesity compared to men and, even in this case, the condition is strongly associated with age.60 It has been hypothesized that sarcopenic obesity is associated with increased fatty infiltration of muscle. Fatty infiltration of skeletal muscle is associated with reduced strength and functional status, and it has been hypothesized that infiltration affects muscle function.61,62 These findings suggest a role of fat mass in the cause of sarcopenia.
Mitochondrial Dysfunction
Mitochondrial function is affected by the cumulative damage to muscle mitochondrial DNA (mtDNA) observed with aging.63 This results in a reduction of the metabolic rate of muscle cell protein and adenosine triphosphate synthesis and, finally, to the death of the muscle fibers and loss of muscle mass.64 Probably, low physical activity is an important contributor for mitochondrial dysfunction in older adults. The decline in mitochondrial function with aging might be attenuated by physical activity.65 some have reported that mitochondrial impairment is only partially reversed after physical training, but does not reach the level of improvement observed in younger adults.66,67
Apoptosis
Accumulated mutations in muscle tissue mtDNA are associated with accelerated apoptosis of myocytes, and apoptosis may also be the link between mitochondria dysfunction and loss of muscle mass.64 Evidence has suggested that myocyte apoptosis is a basic mechanism underlying sarcopenia, and muscle biopsies of older adults show differences associated with apoptosis compared with younger adults.68 It has also been suggested that type II fibers—those fibers preferentially affected by sarcopenia—may be more susceptible to death via the apoptotic pathway.69
Genetic Influences
Genetic factors are major contributors to variability in muscle strength and likely contribute to susceptibility to sarcopenic agents. Genetic epidemiologic studies have suggested that between 36% and 65% of an individual’s muscle strength,70 57% of lower extremity performance,71 and 34% of the ability to perform the activities of daily living72 might be explained by heredity. Sarcopenia and poor physical performance in older adults are also associated with birth weight in men and women, independent of adult weight and height, which suggests that exposure very early in life may also affect risk for sarcopenia in old age in genetically susceptible individuals.73
Few studies have explored potential candidate genes that determine muscle strength. In an analysis of the myostatin pathway, a possible muscle mass regulator, linkage was observed to several areas. Several genes were implicated as positional candidate genes for lower extremity muscle strength.74,75 The actinin–alpha-3 (ACTN3) R577X genotype is of interest because it has been shown to influence knee extensor peak power in response to strength training, as has a polymorphism in the angiotensin-converting enzyme (ACE) gene.76,77 Also, polymorphisms in the vitamin D receptor (VDR) may be associated with muscle strength because of the relationship between vitamin D and its known effect on smooth and striated muscle.48 Polymorphisms in the VDR have been associated with sarcopenia in older men,78 muscle strength and body composition in premenopausal women,79 and muscle strength in older women.80
Low Nutritional Intake and Low Protein Intake
Muscle protein synthesis rate is reported to be reduced by 30% in older adults, but there is controversy about how much this reduction is due to nutrition, disease, or physical inactivity, rather than aging.81,82 It has been recognized by some that protein intake in older adults should exceed the 0.8 g/kg/day recommended intake.83 Muscle protein synthesis is also decreased in fasting older individuals, especially in specific muscle fractions such as mitochondrial proteins,84 and thus the anorexia of aging and its underlying mechanisms contribute to sarcopenia by reducing protein intake.
Evidence-based recommendations for optimal protein intake by older adults were recently released by the PROT-AGE Study Group, an international study group endorsed by several scientific organizations and societies.85 It was noted that to help older adults maintain and regain lean body mass and function, the average daily intake of proteins should be at least in the range of 1.0 to 1.2 g/kg body weight (BW)/day. The group also reinforced the benefits of endurance and resistance-type exercises at individualized levels, suggesting even higher protein intake (≥1.2 g/kg BW/day) for particularly active individuals. The presence of acute or chronic disease, except for severe kidney disease not treated with dialysis, should not prevent the adequate intake of proteins, but should be considered as another reason for increasing the recommended amount of proteins to consume daily.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







