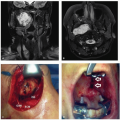
sarcomas particularly difficult. Biopsy sites should be planned such that they can be easily excised during subsequent surgical resection. Pathologic assessment of the biopsy specimen should be undertaken by a pathologist with experience in sarcomas13 with the goal of providing information on the histologic subtype as well as tumor grade at a minimum. Standard morphologic assessment remains the standard for diagnosis, although the use of cytogenetics to augment standard morphologic assessment is accelerating (Table 27.1). For consistency and communication among providers, pathologic reports should use the nomenclature for sarcomas standardized by the WHO.3 Approximately one-quarter of head and neck sarcomas arising in adults are unclassified and undifferentiated, high-grade, pleomorphic sarcomas, with osteosarcomas, angiosarcomas, and rhabdomyosarcomas accounting for another one-third (Table 27.3). However, in children and adolescents, the majority are rhabdomyosarcomas (Table 27.4).
Table 27.1 Etiologic Factors for Head and Neck Sarcomas | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Given the frequent need for adjuvant chemotherapy and radiation therapy either before or after surgery, close coordination with medical and radiation oncologists with experience treating sarcomas is critical.
Table 27.2 Histologic Subtypes of Head and Neck Sarcomas with Known Genetic Alterations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
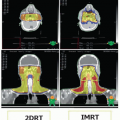
Consequently, preoperative radiation therapy has the advantages of a lower dose (50 Gy vs. 60 to 66 Gy for postoperative radiation therapy) and a smaller, precisely defined radiation field, translating into a lower risk of late radiation-related side effects, including fibrosis and edema, and the potential to minimize the radiation dose to critical structures.23 However, preoperative radiation therapy has been reported to be associated with increased wound complications following surgery. In a randomized trial that attempted to address this issue, 190 patients with soft tissue sarcoma of the extremities were randomized to preoperative or postoperative adjuvant radiation therapy.24 Similar rates of local control, disease-free survival, disease-specific survival, and overall survival were noted between the groups. Among patients with lower extremity sarcomas, wound complications were more common in patients undergoing preoperative radiation therapy. While late side effects of radiation therapy are presumed to be lower with lower doses used preoperatively then with postoperative doses, long-term side effects have not been reported for this trial. In a separate analysis of 40 consecutive patients with head and neck soft tissue sarcomas treated with preoperative radiation therapy, the rate of wound complications was lower than that reported previously for soft tissue sarcomas of the extremities,25 suggesting that the improved blood supply of the head and neck may reduce the risk of some of the wound complications associated with preoperative radiation therapy at other sites. The use of preoperative radiation therapy should be considered in all cases of sarcoma of the head and neck where radiation will be used and the complication risk for surgery in an irradiated field does not preclude it.26 This requires treatment plan coordination and discussion between the treating radiation oncologist and the head and neck surgeon.
Table 27.3 Adult Head and Neck Sarcomas at the University of Texas MD Anderson Cancer Center (Age ≥ 18, 1970-2013) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
less well defined. The Sarcoma Meta-analysis Collaboration analyzed results from 14 trials evaluating doxorubicin-based therapy in resectable soft tissue sarcomas. Local, distant, and overall recurrence-free survival times were significantly improved with doxorubicin-based adjuvant chemotherapy, although the absolute improvement in overall recurrence-free survival was only 6% to 10% at 10 years.27 Other trials have demonstrated histologic subtype-specific responses to different chemotherapeutic agents and are discussed below. Authors increasingly describe the use of chemotherapy to treat sarcomas, particularly the use of neoadjuvant chemotherapy for high-risk tumors.
Table 27.4 Pediatric Head and Neck Sarcomas at the University of Texas MD Anderson Cancer Center (Age < 18, 1970-2013) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
in up to half of patients and portend a worse prognosis.30 Regional nodal disease at presentation is relatively uncommon, occurring in 6% to 10% of patients,31 although up to 16% of patients may experience delayed regional recurrence following treatment.30 The development of distant metastatic disease occurs in 36% to 77% of patients,30,31,32 and the most common site of metastasis is the lungs. Local relapse occurs in 35% to 53% of patients30,31,32 and is more common with larger tumor size, tumor location on the scalp, and single-modality treatment.30,33,34 Local relapse portends a poor prognosis.30
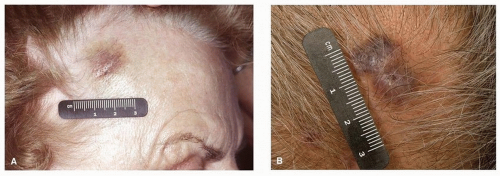 Figure 27.1. Angiosarcoma of the right temple (A) and scalp (B). Early lesions may mimic that of benign conditions such as bruising, leading to delays in diagnosis. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree