Key Points
- 1.
Uterine sarcomas are uncommon tumors. The most common tumors are leiomyosarcomas (LMSs) (59%) and endometrial stromal sarcomas (ESSs) (33%). Other sarcomas comprise less than 8% of the remaining.
- 2.
The standard treatment for any sarcoma is removal of the uterus tubes and ovaries if possible.
- 3.
The prognosis of LMSs is poor; even at an early stage, adjuvant therapy has not been shown to affect survival.
- 4.
ESSs are often hormonally responsive.
Classification
Sarcomas are uncommon tumors arising from mesenchymal elements and are distinguished from carcinomas that arise from epithelial elements. Uterine sarcomas are thought to arise primarily from two tissues: endometrial stroma and the uterine muscle itself. When endometrial mesenchymal tissue undergoes malignant transformation, it may be accompanied by a malignant epithelial component (carcinosarcoma [CS], formerly referred to as malignant mixed Müllerian tumor), it may be associated with a benign-appearing epithelial component (adenosarcoma), or it may have no recognizable epithelial component (endometrial stromal sarcoma [ESS]). Tumors arising from malignant transformation of uterine smooth muscle are known as uterine leiomyosarcomas (LMSs). Other sarcomas, such as angiosarcoma and fibrosarcoma, arise in supporting tissues and are rare.
Incidence and Epidemiology
The Surveillance, Epidemiology, and End Results (SEER) database notes that LMSs account for 59% of 8365 cases reported. ESSs are represented by 33%, and other sarcomas account for 8%. The peak age for all sarcomas was 45 to 54 years with the peak age for LMS being slightly older than 55 years.
In previous editions, classification of sarcomas included CS, LMS, and ESS as the three most common uterine sarcomas with CS being the most common. Convincing evidence now suggest that CSs are not true sarcomas but are derived from epithelial cells and therefore are now included as a type 2 endometrial cancer category. Although there are minor other types of uterine sarcoma, their occurrence is such that they will not be included in this chapter.
Sarcomas arising within the uterus are relatively rare and account for 3% to 8% of uterine cancers. According to the SEER, the age-adjusted incidences for all sarcomas (per 100,000 women age 35 years and older) in US women were 2.68 for Native American, Asian, and Hispanic; 3.58 for white; and 7.02 for black women. By comparison, the incidence for epithelial uterine cancers, per 100,000 women, is roughly nine for black women and 20 for white women. Uterine sarcomas represented 8% of primary uterine malignancies in the most recent analysis of the SEER database. Harlow and coworkers had previously reported from SEER databases covering 1973 to 1981 that suggested an annual incidence of only 1.7 cases per 100,000 women. Sarcomas have been traditionally thought to represent only 3% to 5% of all uterine tumors. The increasing incidence of uterine sarcomas noted in the SEER studies may reflect better diagnosis and perhaps a true increase in an aging population.
The type and frequency of uterine sarcomas are related to both age and race. LMS can occur at an early age, has an incidence plateau in middle age, and declines thereafter. In a large prospective surgical-pathologic study conducted by the Gynecologic Oncology Group (GOG) evaluating patients with all types of sarcomas, the median age of patients with LMS was 55 years compared with 65 years for those with CS. Brooks and coworkers suggested that white women were older at the time of diagnosis of their sarcomas compared with black women.
Using SEER data (1992–1998), Sherman and Devesa reported on racial differences in uterine malignancies. They found that for all histopathologic categories, the age-adjusted incidence of uterine cancers (per 100,000 women) was 23 for non-Hispanic white, 14 for white Hispanic, and 15 for black women. In contrast, LMSs are more common in black women. For LMSs, ESSs, and adenosarcomas combined, the incidence was 1.24 for black versus 0.79 for non-Hispanic white women. Harlow and associates found the same trend reporting on an earlier SEER data set. It has also been suggested that blacks present with stage I disease less commonly than whites. Given that uterine sarcomas are rare and form a heterogeneous group, little is known about other risk factors favoring development of these tumors.
Leiomyosarcoma
Clinical Profile
Many of these patients experience perimenopausal bleeding, are found to have a pelvic mass on examination, and will be thought to have uterine leiomyomas. Giuntoli and coworkers, reporting on the Mayo Clinic experience of 208 patients with uterine LMS collected over a 23-year period, found that vaginal bleeding was the most common symptom (56%) followed by a palpable pelvic mass (54%) and pelvic pain (22%). A commonly described “clinical pearl” has been the relationship of a rapidly enlarging uterus to LMS. The data to support such an observation are mixed, however . Parker and associates evaluated 1332 patients who underwent surgery for presumed leiomyoma. In the group of 371 patients who had rapid uterine growth, only one case (0.2%) of LMS was identified. Similarly, in a subgroup of 198 patients who had carefully documented rapid uterine growth, no cases of LMS were found. Leibsohn and coworkers reported on 1432 patients undergoing hysterectomy for bleeding related to uterine leiomyomas and identified 7 (0.49%) patients with LMS.
Because LMS arises within the uterine smooth muscle, biopsy of the malignant tissue is difficult, and many lesions are found only at final pathology. In Leibsohn et al.’s series none of the seven patients with LMS were identified on preoperative biopsy and in only three cases was there an intraoperative suspicion of sarcoma. Various authors have reported that LMS may be present in the submucosa of the uterus in 30% to 50% of patients, but even at that, biopsy diagnosis is not easily accomplished. Schwartz and coworkers described the tumors to be both broad based and pedunculated and that in 19 of 20 cases, the LMS was confined to one mass.
Because of the difficulty in establishing a preoperative diagnosis and the high frequency of uterine leiomyoma in the population, it is not unexpected that several case reports have detailed the finding of LMSs in patients who have undergone conservative management of symptomatic leiomyomas by myomectomy, after Lupron treatment before myomectomy, and after vascular embolization of presumed leiomyomas. These cases speak to the importance of pretreatment counseling of patients who undergo such therapies.
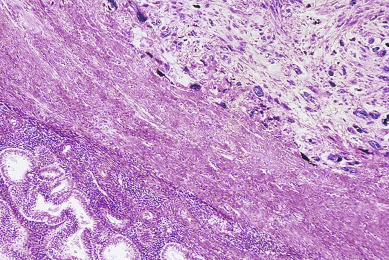
There is considerable discussion about the histologic criteria necessary for the diagnosis of LMS ( Fig. 6.1 ). LMSs must be distinguished from a variety of benign smooth muscle tumors ( Table 6.1 ). The predominant differentiating features between benign and malignant tumors include mitotic activity (as gauged by the number of mitotic figures per 10 high-power fields [hpf]), cellular atypia, and necrosis. Leiomyoma, cellular leiomyoma, and bizarre leiomyoma (also called atypical or symplastic leiomyoma) are considered to be benign. These entities are distinguished from LMS mainly by the mitotic count of the tumor. Although cellular leiomyomas and bizarre leiomyomas may appear at first sight to be malignant, they contain fewer than 5 mitoses/10 hpf on histologic evaluation, and the prognosis is excellent with surgery only. Smooth muscle tumors of uncertain malignant potential (STUMP) include a group of smooth muscle tumors with 5 to 9 mitoses/10 hpf that can exhibit a variable behavior.
| Mitotic Figures/10 hpf | Atypia * | Diagnosis | Metastatic Potential |
|---|---|---|---|
| 1–4 | Any degree | Leiomyoma | Very low |
| 5–9 | None | Leiomyoma with high mitotic activity | Very low |
| 5–9 | Grade 1 | Smooth muscle tumor of uncertain malignant potential | Low |
| 5–9 | Grade 2 or 3 | Leiomyosarcoma | Moderate |
| ≥10 | Grade 1 | Leiomyosarcoma | High |
| ≥10 | Grade 2 or 3 | Leiomyosarcoma | Very high |
Intravenous leiomyomatosis is a rare smooth muscle tumor characterized by nodular masses of histologically benign smooth muscle cells growing within venous channels that are lined by epithelium; arteries are not involved. Treatment involves surgical removal, and the prognosis is good. Recurrences are unusual and are usually managed successfully with further surgical excision.
Benign metastasizing leiomyoma is another rare condition in which smooth muscle tumor deposits are found in the lung, lymph nodes, or abdomen and appear histologically like a benign leiomyoma. Most women have a history of pelvic surgery for benign leiomyomas years before these metastatic sites are recognized. Surgical excision has been the primary treatment.
Norris and Taylor believed that mitotic count was extremely important in that if fewer than 10 mitoses/10 hpf were identified, the lesion was benign regardless of the degree of cellular atypia; if more than 10 mitoses/10 hpf were present, the prognosis was grave. More recently, Norris and Taylor stated that tumors with fewer than 5 mitoses/10 hpf can rarely metastasize. In a follow-up study from the Armed Forces Institute of Pathology, O’Connor and Norris evaluated 73 smooth muscle tumors of the uterus with 5 to 9 mitotic figures/10 hpf but lacking cytologic atypia. They concluded that the metastatic rate was too low to consider these as being sarcoma. Several of their patients were treated only with myomectomies with excellent results. Lissoni and colleagues have suggested extending this philosophy to additional patients.
Kempson and Bari believe that the mitotic count is important but state that prognosis is poor if more than 5 mitoses/10 hpf are identified. Their experience with tumors containing 5 to 9 mitoses/10 hpf indicates that the tumors usually behave aggressively and will metastasize. These authors believe that the degree of cellular atypia is of limited value by itself in determining the malignancy of smooth muscle tumors. In tumors with higher mitotic counts, there were usually a greater number of very atypical cells. This atypia was also seen in tumors with 5 to 9 mitoses/10 hpf. Tumors with fewer than 5 mitoses/10 hpf were thought to be benign regardless of the atypia of the cells. None of Kempson and Bari’s patients with fewer than 5 mitoses/10 hpf had disease outside the uterus, but distant disease was a common finding if more than 5 mitoses/10 hpf were noted. The presence of coagulative necrosis, especially with diffuse significant atypia, suggests strongly that the lesion is a LMS regardless of the mitotic count.
However, Silverberg believes that the mitotic count alone cannot be used as a strict histologic criterion because he had patients with fewer than 10 mitoses/10 hpf who succumbed to their disease. He emphasized that the grade of the tumor, which reflects the cytologic atypia, is a better criterion than mitotic count alone. Toledo and Oliva point out that mitotic rate alone is not sufficient to predict prognosis. Although mitotic rate and nuclear atypia are important features, tumor cell necrosis is a unique feature of LMS. Most uterine LMS with necrosis will show high mitotic rate and atypia. Essentially all investigators note the gravity of the situation if intravascular invasion or disease outside the uterus is found. Silverberg believes that the single most important prognostic indicator is the menopausal status of the patient. Women who are premenopausal when the diagnosis is made tend to have a much better prognosis than that of women who are postmenopausal, even when criteria such as blood vessel invasion, growth pattern, grade, and mitotic counts are considered. LMSs occur in young patients and tend to be more localized when they are first diagnosed, and they probably exhibit a slower growth pattern than CSs or ESSs do.
Surgical Management
Planning the surgical management of LMS is difficult because many cases go unrecognized preoperatively. Patients commonly undergo myomectomy or hysterectomy for presumptive leiomyomas, which are subsequently identified as a sarcoma. When a preoperative diagnosis is known, hysterectomy should be performed. Retention of the ovaries in premenopausal patients has not shown to worsen outcome in several retrospective series and may be considered. Surgical staging with lymph node dissection is controversial, but most authorities tend to recommend biopsy of suspicious nodes only. When LMS is recognized postoperatively, reexploration for the purposes of completing surgical staging is not recommended. After diagnosis, evaluation of the chest with chest radiography or computed tomography is reasonable given the propensity of spread to the lungs. Goff and colleagues found that 10% of patients with LMS had lung metastases at presentation.
Data on patterns of spread for LMS are limited by the rarity of the tumor. Goff and coworkers found that 16 of 21 patients had stage I disease at surgery, and only patients with disseminated intraabdominal disease had nodal involvement. Giuntoli and coworkers reported that only 34 of 208 patients in the Mayo Clinic series had pelvic nodal dissections performed, and of the four with positive nodes, extrauterine disease was reported in three. The GOG sarcoma study on patterns of spread found that in 59 surgically staged patients, 5% had positive extrauterine spread to peritoneal cytology, 3% had adnexal involvement, and 3.5% had nodal metastases ( Table 6.2 ). After surgical staging, 83% were stage I, and only 13% of patients were upstaged based on biopsies. In 2009, the Federation of International Gynecologists and Obstetricians (FIGO) introduced a new staging system for LMS, ESS, and adenosarcoma. For LMS, stage reflects tumor size and extrauterine spread (see Table 6.2 ).
| Stage I (tumor limited to uterus) * |
| IA: <5 cm tumor size |
| IB: ≥5 cm tumor size |
| For Leiomyosarcoma |
| IA: tumor limited to endometrium or endocervix without myometrial invasion |
| IB: tumor invades <50% myometrium |
| IC: tumor invades ≥50% myometrium |
| For Endometrial Stromal Sarcoma and Adenosarcoma |
| Stage II (tumor extension into the pelvis) |
| IIA: adnexal involvement |
| IIB: other extrauterine pelvic disease |
| Stage III (tumor invades abdominal tissues) |
| IIIA: one site of involvement |
| IIIB: >1 site of involvement |
| IIIC: metastasis to pelvic and/or paraaortic lymph nodes |
| Stage IV (tumor invades bladder and/or rectum, and/or distant metastasis) |
| IVA: involvement of bladder and/or rectum |
| IVB: distant metastasis |
* For stage I disease, two substages are used based on tumor type.
Leiomyosarcomas most commonly spread hematogenously. Corscaden and Singh reported the results of autopsies of 15 patients who died of LMS of the uterus. Of these patients, 100% had intraabdominal visceral involvement, 80% had lung or pleural metastases, 40% had paraaortic nodal involvement, 33% had renal metastases, and 20% had liver metastases. In the GOG study, the most common first site of recurrence was the lung (41%), and only 13% had a pelvic failure ( Table 6.3 ).
| Carcinosarcoma (%) ( n = 301) | Leiomyosarcoma (%) ( n = 59) | Endometrial Adenocarcinoma (%) ( n = 621) | |
|---|---|---|---|
| Deep myometrial invasion | 37 | — | 22 |
| Positive peritoneal cytology | 21 | 5 | 12 |
| Adnexal involvement | 12 | 3 | 5 |
| Nodal metastases | 17 | 3.5 | 9 |
The prognosis for LMS is poor, even for early-stage disease. Vardi found that of the total group of 32 patients, 44% died of disease within the first 3 years after diagnosis. There was a 63.6% 5-year survival rate in women in whom diagnosis was made while they were premenopausal compared with a 5.5% 5-year survival in postmenopausal women. The GOG found that only 31% of patients remained disease free at 3 years. Gadducci and coworkers found that 39% of patients with stage I or II disease had a recurrence, with a median time to recurrence of 18 months. Berchuck and coworkers reported that only 29% of patients with stage I or II disease remained free of disease, with a median follow-up period of 7.5 years. Although almost all deaths and recurrences are during the 4 years after diagnosis, Gallup and coworkers reported a recurrence 25 years after initial therapy. Contrary to an older perception, these data indicate that LMSs have a poorer prognosis than CSs.
Predictors of outcome have been assessed by several groups. The GOG found that patients with greater numbers of mitoses were associated with an increased risk of recurrence such that 79% of patients with more than 20 mitoses/hpf recurred compared with 61% with 10 to 20 mitoses/hpf. Patients who present with extrauterine disease also have a very poor prognosis. Berchuck and coworkers found no survivors beyond 2 years in this group of patients. Gadducci and coworkers assessed 126 patients collected from a multi-institutional study and identified stage, mitotic count, and age as independent prognostic factors predicting recurrence. Giuntoli and coworkers found that high grade, advanced stage, and having had ovaries removed at surgery were independent predictors of poorer survival.
Adjuvant Therapy
For patients with LMS, no adjuvant therapy has been shown to be effective in prolonging survival. As with other high-risk uterine cancers, LMSs have been managed postoperatively by radiation therapy or chemotherapy. Berchuck and coworkers found that among patients receiving any form of adjuvant therapy, 83% recurred compared with 68% who underwent surgery alone. Given the propensity for hematogenous spread, radiation does not adequately address the high frequency of distant sites of failure (lung, liver, abdominal cavity). Supporters of radiation note that pelvic control may be obtained, which can prevent bulky pelvic recurrences and improve patient comfort and quality of life. The GOG reported that only three of 13 patients who received radiation remained without recurrence, but no pelvic failures were seen. Giuntoli and coworkers performed a subset analysis within their large series of patients, identifying 31 patients who received adjuvant radiation therapy. In a comparison with 31 well-matched patients who did not receive radiation, there was not a statistically significant difference in survival between the groups, although the 5-year survival rate for those receiving radiation was 60% compared with 40% without radiation therapy. In the only prospective randomized trial, the European Organization for Research and Treatment of Cancer (EORTC) showed in the subgroup of 103 patients with LMS, there was no improvement of local control with radiation (local recurrence; 20% after radiation vs. 24% after surgery alone). Of note, most patients developed distant sites of failure.
Given the importance of distant failures in this disease, chemotherapy has been used to manage early-stage LMSs. In the GOG adjuvant trial evaluating the role of doxorubicin with or without radiation, 61% of patients with LMS treated without doxorubicin compared with 44% who received chemotherapy had a recurrence. This study was too small to specifically evaluate the importance that histology played in response to therapy. With the identification of an active combination regimen of docetaxel plus gemcitabine for patients with advanced or recurrent disease, early data suggest that this regimen may hold promise in an adjuvant setting . In one series of 18 patients with stage I or II LMS, 59% remain progression free at 3 years.
Hensley et al. reported results of a phase II study of 23 patients completely resected LMS treated with four cycles of gemcitabine and docetaxel. In patients with uterus-limited disease, the 2-year progression-free survival (PFS) rate was 59%. GOG 277 evaluated 47 women with uterine-limited disease with four cycles of doxorubicin followed by four courses of gemcitabine and docetaxel. At a median follow-up period of 27.4 months, a 78% 2 year PFS was noted with a median PFS of 39.3 months.
Hensley and coworkers more recently enrolled 46 with resected uterus-limited high-grade uterine LMS who received four cycles of gemcitabine and docetaxel. If confirmed disease free after four cycles, they then received four cycles of doxorubicin. At 2 years, PFS was 78% of patients; it was 57% at 3 years. Bevacizumab was added to the gemcitabine–docetaxel regimen in 25 women with combined complete and partial response of 44%. GOG 250 enrolled patients with LMS in a phase III placebo-controlled study to evaluate the addition of bevacizumab to gemcitabine and docetaxel. The addition of bevacizumab failed to improve PFS, overall survival, or OR. Currently, there is a randomized trial of adjuvant chemotherapy versus observation in women with LMS limited to the uterus (GOG 277).
Multiple single agents have been tried and have been unsuccessful. Recently, there has been an increased interest in the study of biologic targets, including mTOR (mammalian target of rapamycin) inhibitors, with modest results. Multikinase inhibitors that affect the vascular endothelial growth factor pathway to date have been unsuccessful.
Management of Recurrent Disease
Data from randomized phase III trials conducted by the GOG, which included advanced and recurrent uterine sarcomas of all types, helped to identify the differential sensitivity of LMSs compared with CSs to different agents. In the trial comparing doxorubicin with and without dacarbazine (DTIC), patients with LMSs were found to have longer survival times compared with patients with other types when treated with a doxorubicin-containing regimen. In a subsequent study using doxorubicin with or without cyclophosphamide, histologic type was not found to be a prognostic indicator. Given that 80% of all patients died of disease within 2 years, the GOG adopted a strategy of performing phase II trials with the hope of identifying active agents that could later be tested in randomized studies. The rarity of LMSs, however, has made randomized study of this tumor more difficult.
Historically, doxorubicin and ifosfamide have demonstrated the most activity in patients with recurrent disease with responses ranging from 25% to 33% for doxorubicin and 18% for ifosfamide when used as single agents. Sutton et al. and the GOG published the results of a phase II study combining ifosfamide and mesna and doxorubicin, showing an overall response rate of 30%. The duration response was 4 months, and the regimen produced substantial toxicity. Paclitaxel has been studied in LMSs, with an 8% response seen in patients who had received prior chemotherapy and a 9% response in those who were chemotherapy naïve. A novel combination of gemcitabine with docetaxel has shown a 53% response rate in a small phase II trial of patients that included 29 patients with uterine LMS. This regimen was subsequently evaluated in two phase II trials. In one trial of 39 patients with advanced or recurrent LMS who had received no prior chemotherapy, the regimen produced a 36% response rate and a median PFS of 4.4 months. In a similar population of 48 patients who had received one prior chemotherapy regimen, a 27% response rate was noted. In both studies, myelosuppression was the most common toxicity.
Targeted biologic agents are also being explored, although no agent has yet to demonstrate activity in this tumor type. A randomized trial comparing the docetaxel plus gemcitabine regimen with or without the antivascular endothelial growth factor antibody bevacizumab is currently ongoing. Patients with late recurrences of LMS in the form of isolated pulmonary metastases are candidates for thoracotomy and sequential resection of the lesions. Five-year survival rates of 30% to 50% have been reported after such therapy.
Recently, several reports have appeared in the medical as well as lay literature in which women with a preoperative diagnosis of leiomyoma were treated with minimally invasive surgery including morcellation, and the final pathology noted a uterine malignancy. Most of these cancers were LMS. Because preoperative diagnosis of LMS is very imprecise compared with other uterine malignancies and occur in peri- or early menopause, a benign preoperative diagnosis could be easily made. These cases have resulted in disseminated disease with poor prognosis. As a result, several studies have evaluated the incidence of malignancy associated with a hysterectomy or myomectomy done for presumed benign disease. In reported studies of women undergoing hysterectomy or myomectomy for a myometrial mass, the prevalence of sarcoma is about 1 in 500 (0.2%) in most studies and reviews. Most sarcomas were LMS. The largest study of unexplained malignancy in women undergoing morcellation was from the US insurance database including 232,882 women undergoing minimally invasive hysterectomy noted morcellation was performed in 36,470 (15.7%). Among those in which morcellation was performed, 99 cases (0.34%) of uterine cancer were found. The prevalence of cancer in this series for women younger than the age of 40 years was 1 in 1500 and 1 in 1100 for women 40 to 44 years of age. Unfortunately, histology of uterine cancer cases was not reported. Data from a national US hospital database of 41,777 women undergoing myomectomy included 3220 who underwent power morcellation, and 0.09% had uterine cancer. Occult uterine cancer was 1 in 2337 in women younger than 40 years, 1 in 702 in those age 40 to 49 years, 1 in 154 in those age 50 to 59 years, and 1 in 31 in women age 60 years or older. Studies have noted morcellation is associated with the worst prognosis with uterine sarcoma. A meta-analysis of four observational studies in women with uterine sarcoma found morcellation compared with no morcellation was associated with higher recurrence rate (61% vs. 39%) and mortality rate (48% vs. 29%). These data support the US Food and Drug Administration guideline that power morcellation should not be used in peri- and postmenopausal women (November 2014).
More recent data by Wright et al. using a Markov model suggest that morcellation may be beneficial in women younger than the age of 40 years and not beneficial in older women. Contained morcellation in a containment bag is a potential emerging technique that may put this controversy to rest.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree