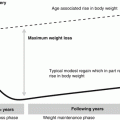
Fig. 14.1
Pathways of steroid metabolism. A block due to deficiency of 21-hydroxylase causes accumulation of precursors (17-hydroxyprogesterone, androstenedione, testosterone) and deficiency of glucocorticoids (cortisol) and mineralocorticoids (aldosterone)
Classical 21-Hydroxylase Deficiency Congenital Adrenal Hyperplasia
The incidence of congenital adrenal hyperplasia in Britain is about 1 in 18,000 [1]. Up to 95 % of cases are caused by deficiency of the 21-hydroxylase enzyme; this results from mutations in the gene encoding adrenal P450c21 and is inherited in an autosomal recessive fashion. Complete deficiency of the enzyme results in salt wasting due to inability to produce the mineralocorticoid aldosterone, and hypoglycaemia from glucocorticoid deficiency. Due to the position of 21-hydroxylase in the steroid pathway, the block causes accumulation of precursors which are diverted into the alternative pathway producing excess 17 hydroxyprogesterone, which is converted to androstenedione and thence to testosterone, both potent androgens. Females therefore present with virilisation at birth, which can vary from mild clitoromegaly to complete gender ambiguity. As they are diagnosed within the first few days of life, they rarely present with salt losing crises at diagnosis (although they are just as prone as boys with this condition to decompensate during intercurrent illnesses or other times of physical stress), but the birth of a child with genital ambiguity (disorder of sex development) is an extremely traumatic experience for the parents and great sensitivity is required in making an accurate and timely diagnosis.
Careful examination will usually show clitoromegaly, enlarged or fused labia majora, bifid or completely hypoplastic scrotum with no palpable gonads in the labioscrotal folds. Only a single perineal orifice will be seen as vagina and urethra are often fused (urogenital sinus). If faced with this situation, an urgent pelvic ultrasound should be arranged: in a girl with virilising CAH a uterus and ovaries should be visible and will immediately help to differentiate between CAH and other conditions causing disorders of sex development. A rapid karyotype will be 46 XX and biochemical confirmation can be obtained (after 48 h of age to avoid the physiological surge of maternal hormones) by checking adrenal function (17-OHP, androstenedione, cortisol, DHEAS, testosterone) and plasma renin activity. A urinary steroid profile and DNA analysis for the 21-hydroxylase gene mutation will confirm the diagnosis.
As external virilisation in boys with this condition is not a problem, boys present between 7 and 21 days, when the baby switches from the protection of maternal hormones and relies on his own adrenal glands. Lack of mineralocorticoid precipitates salt loss which, uncorrected and in combination with glucocorticoid deficiency, precipitates an adrenal crisis. Typically this presents as poor feeding, lethargy and weight loss. Vomiting can follow with collapse if not picked up early enough. After the diagnosis has been made and the child stabilised, mutation analysis can confirm the genetic basis of the condition and allow appropriate counselling for parents, who, as this is often their first child, will want to know of recurrence risks in future pregnancies (one in four with every pregnancy). A full multidisciplinary team comprising endocrinologists, geneticists, urologists, psychologists, gynaecologists and clinical nurse specialists needs to be involved so that the parents can be supported practically and psychologically [2]. Even after diagnosis and appropriate treatment has been instigated, infants remain very vulnerable to recurrent salt-losing crises and hospital admissions, due to their propensity to contract repeated infections, and parents must be taught how to manage illnesses at home and be given direct access to paediatric wards if their child’s condition deteriorates.
In the longer term, girls with CAH will require surgery which may be minor when virilisation is not extensive, to major reconstruction with vaginoplasty/ feminising genitoplasty, sometimes followed by self-vaginal dilatation. Boys usually do not require surgery, but complications in both can include obesity, precocious puberty, short stature and subfertility, with development of polycystic ovary syndrome or adrenal rest tumours [3].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree