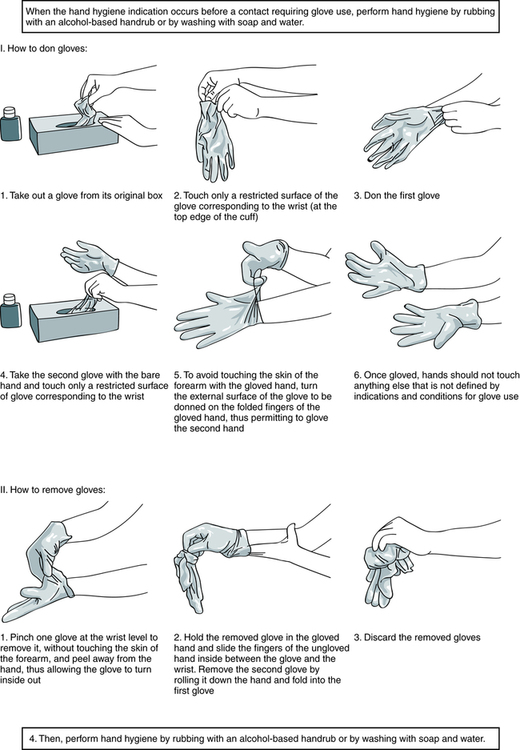
At the conclusion of this chapter, the reader should be able to: • Name the federal or national agencies responsible for safety issues. • Discuss the occupational transmission of hepatitis B virus (HBV) and human immunodeficiency virus (HIV). • Describe the practice of Standard Blood and Body Fluid Precautions. • Explain the proper handling of hazardous material and waste management, including infectious waste, chemicals, and radioactive waste. • Describe the basic aspects of infection control policies, including the use of personal protective equipment or devices (gowns, gloves, goggles) and the purpose of Standard Precautions. • Compare preexposure and postexposure prophylactic measures for handling potential occupational transmission of certain pathogens (HBV, HCV, HIV). • Demonstrate the proper decontamination of a work area at the start and completion of work and after a hazardous spill. • Explain the process of properly segregating and disposing of various types of waste products generated in the clinical laboratory. • Analyze a safety case study to identify violations and remediations for the violations. • Correctly answer case study related multiple choice questions. • Be prepared to participate in a discussion of critical thinking questions. • Identify items essential to safety in the clinical laboratory. • U.S. Department of Labor, Occupational Safety and Health Administration (OSHA) • Clinical and Laboratory Standards Institute (CLSI), a nonprofit educational organization that provides a forum for the development, promotion, and use of national and international standards • Centers for Disease Control and Prevention (CDC), part of the U.S. Department of Health and Human Services Public Health Service • College of American Pathologists (CAP) 1. Staff must wear laboratory coats and be additionally protected from contamination by infectious agents. 2. Food and drinks should not be consumed in work areas or stored in the same area as specimens. Containers, refrigerators, or freezers used for specimens should be marked as containing a biohazard. 3. Specimens needing centrifugation are capped and placed into a centrifuge with a sealed dome. 4. A gauze square is used when opening rubber-stoppered test tubes to minimize aerosol production (introduction of substances into the air). 5. Autodilutors or safety bulbs are used for pipetting. Pipetting of any clinical material by mouth is strictly forbidden. • Educate and train all health care workers in Standard Precautions and in preventing bloodborne infections. • Provide proper equipment and supplies (e.g., gloves). • Concentration of HBV or HIV; viral concentration is higher for HBV than HIV • Presence or skin lesions or abrasions on the hands or exposed skin of the health care worker • Percutaneous (parenteral) inoculation of blood, plasma, serum, or certain other body fluids from accidental needlesticks • Contamination of the skin with blood or certain body fluids without overt puncture, caused by scratches, abrasions, burns, weeping, or exudative skin lesions • Exposure of mucous membranes (oral, nasal, or conjunctival) to blood or certain body fluids, as the direct result of pipetting by mouth, splashes, or spattering • Centrifuge accidents or the improper removal of rubber stoppers from test tubes, producing droplets. If these aerosol products are infectious and come into direct contact with mucous membranes or nonintact skin, direct transmission of virus can result. General guidelines related to the selection and general use of gloves include the following: 1. Use sterile gloves for procedures involving contact with normally sterile areas of the body or during procedures in which sterility has been established and must be maintained. 2. Use nonsterile examination gloves for procedures that do not require the use of sterile gloves. Gloves must be worn when receiving phlebotomy training. The National Institute of Occupational Safety and Health mandates the use of gloves for phlebotomy. 3. Gloves should be changed between each patient contact. 4. Wear gloves when processing blood specimens, reagents, or blood products, including reagent red blood cells. 5. Gloves should be changed frequently and immediately if they become visibly contaminated with blood or certain body fluids or if physical damage occurs. 6. Do not wash or disinfect latex or vinyl gloves for reuse. Washing with detergents may cause increased penetration of liquids through undetected holes in the gloves. Rubber gloves may be decontaminated and reused, but disinfectants may cause deterioration. Rubber gloves should be discarded if they have punctures, tears, or evidence of deterioration or if they peel, crack, or become discolored. 7. Using items potentially contaminated with human blood or certain body fluids (e.g., specimen containers, laboratory instruments, countertops) Care must be taken to avoid indirect contamination of work surfaces or objects in the work area. Gloves should be properly removed (Fig. 6-1) or covered with an uncontaminated glove or paper towel before answering the telephone, handling laboratory equipment, or touching doorknobs.
Safety in the Immunology-Serology Laboratory
Safety Standards and Agencies
Prevention of Transmission of Infectious Diseases
Protective Techniques for Infection Control
Selection and Use of Gloves
Safety in the Immunology-Serology Laboratory