Abstract
Patients with brain and spinal cord malignancies may incur disability due to the effects of tumor or cancer-related treatment, including surgery, chemotherapy, and radiation. It is essential for physiatrists working with cancer survivors to be familiar with common impairments in this population as well as ongoing safety challenges that brain and spinal cord tumor patients may face while undergoing rehabilitation. This chapter discusses common impairments caused by radiation and chemotherapy, both physical and cognitive. Special considerations are given to impairments/treatments that may pose safety challenges such as bone metastases and osteoporosis, cytopenias, the use of steroids and seizure medications as well as neuropathy.
Keywords
Bone metastasis, Cancer rehabilitation, Chemotherapy, Cytopenia, Exercise, Radiation, Safety, Steroids
Introduction
Survivors of brain and spinal cord cancer often experience long-term complications secondary to the disease itself or treatment regimens, which can lead to disability and loss of function. Some of the most common complications in this patient population include cognitive impairments, hemi or complete paresis, and gait and balance disturbances. Approximately 80% of patients with primary brain tumors have an impairment in cognition at some point in the course of their disease. Although variable with location of tumor and type of treatment, individuals with brain tumors have reported the rate of hemiparesis to range from 26% to 47% and gait impairment ranges from 26% to 62%. Individuals with brain and spinal cord tumors also suffer from medical complications such as chemotherapy-induced cytopenia and neuropathy, osteoporosis, steroid-related myopathy and seizure. Awareness of all potential disease and treatment-related complications can help the rehabilitation team to properly care for and safely direct the rehabilitation for this patient population, with the goal of preventing adverse outcomes and maximizing an individual’s functional independence.
Provider Training
Following a multidisciplinary approach to rehabilitation, all clinicians treating those with cancer in the rehabilitation setting require knowledge of the needs specific to this patient population.
Although formal standards of competency required during residency training have yet to be established by the Accreditation Council for Graduate Medical Education (ACGME) or the American Board of Physical Medicine and Rehabilitation (ABPMR), a large number of physiatrists receive an “average” exposure to cancer rehabilitation during residency. The field is also growing and Physical Medicine and Rehabilitation (PM&R) physicians recognize the vital role rehabilitation can play in the care of patients with cancer. At present time, there are five fellowship programs in cancer rehabilitation across the United States, with the hope for more in the future. The role of cancer rehabilitation has been well established and recognized by the American Physical Therapy Association and the association’s work has now led to recognition by the American Board of Physical Therapy Specialties as well.
The Commission on Accreditation of Rehabilitation Facilities (CARF) has outlined the requirements need for a cancer rehabilitation specialty program, which focuses on an individualized, patient-centered, interdisciplinary program for those with cancer. CARF requires cancer rehabilitation programs to demonstrate “competencies and the application of evidence based practices to deliver services that address the preventative, restorative, supportive and palliative rehabilitation needs” of those which it serves. With these guidelines and adequately trained physicians and therapists, a rehabilitation program is able to provide patient-centered care, specializing in the needs of this specific patient population. Communication between healthcare providers is emphasized, as is advocating to the community and legislators on behalf of patients with cancer. The goal of a cancer specialized rehabilitation program is optimizing functional independence and outcomes in an individualized manner, throughout one’s course of illness, treatment, and long-term care.
Engaging Family Members in Patient
Safety
Cancer patients often face seeing multiple specialists across the whole spectrum of care from acute care hospitals to rehabilitation facilities and outpatient practices. Numerous handoffs and transitions may result in miscommunication and errors. Engaging family members and educating them regarding patient’s medications, precautions, and necessary follow-up will ensure patient safety through multiple transitions. In addition, patients may be engaged in learning proper techniques to assist cancer survivors with activities of daily living (ADLs) as they transition to home and community.
Brain and Spine Radiation
Side effects of brain and spine radiation vary with the dose, duration, and amount of brain or spine treated. These side effects may present safety concerns both in the inpatient and outpatient rehabilitation setting. The timeline of common side effects can be divided into three phases: acute, early delayed, and late.
Brain Radiation
Acute complications of brain radiation occur during radiation therapy (RT) and up to 6 weeks after. These effects are often due to vasogenic edema and present as an acute encephalopathy with symptoms including headache, nausea, new onset or worsening of focal neurologic deficits and seizure. The acute side effects are most often seen on the inpatient rehabilitation unit and can present a safety concern, as patients may exhibit decreased safety awareness and increased risk of falls. Fortunately, RT encephalopathy is most often reversible, being easily treated or prevented with corticosteroids. Although often multifactorial, RT can contribute to the fatigue a patient experiences while undergoing treatment. Fatigue can hinder a patient’s performance in therapy and may also increase their risk for falls on the inpatient unit.
Early delayed side effects present from 6 weeks to 6 months post RT and most commonly include persistent fatigue and focal neurologic deficits or encephalopathy, thought to be caused by demyelination of the irradiated tissue. , Similar to the complications seen in the acute phase, early delayed side effects are most often reversible when treated with corticosteroids.
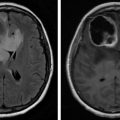
Late effects occur at least 6 months to a year after completion of RT and may present several years after. Unlike the acute or early delayed side effects, these effects are usually irreversible. Radiation necrosis develops with higher fractionation of radiation and is due to damage and necrosis of blood vessels, combined with demyelination of treated and surrounding brain tissue. This type of necrosis leads to new focal neurologic deficits, dementia, and ataxia, all of which present safety concerns for the patient. Incidence of long-term cognitive deficits varies with location of the tumor and fractionation and duration of RT, but may cause memory impairments, decreased concentration, and safety awareness. Distinguishing between radiation necrosis and recurrent tumor is often difficult, as the two have similar clinical presentations. MRI imaging of the brain without a clearly defined T2 mass as well as a higher ratio of edema to enhancing lesion are more suggestive of radiation necrosis than recurrent disease.
Radiation to the Spine
RT required for tumors of the spine may cause damage and fibrosis of the spinal cord itself, as well as the exiting roots and nerves and surrounding musculature. Damage to these structures may present as symptoms of myelopathy, radiculopathy, neuropathy, plexopathy, or myopathies. The rehabilitation team should be aware of structures included in the irradiated field during treatment in order to properly diagnose these potential side effects.
Side effects from RT to the spinal cord presenting a safety risk include transient myelopathy, chronic progressive myelopathy, and acute paralysis. Transient myelopathy, due to demyelination, occurs in the early delayed time frame and presents as Lhermitte symptom, a shock-like sensation down the spine induced by flexion of the neck. This myelopathy, although uncomfortable, resolves with time. Late radiation–induced myelopathy is most often irreversible and can be progressive in nature, leading to paralysis. Demyelination and necrosis of white matter, as well as vascular necrosis all contribute to chronic radiation myelopathy. Initial presentation is often with Brown-Sequard syndrome which then progresses with time to spastic paraplegia or quadriplegia with associated complications such as neurogenic bowel and bladder. Corticosteroids may be prescribed to slow the progression. This progressive myelopathy of course increases a patient’s risk for falls and skin breakdown. Lower motor neuron syndrome may occur with RT to the lower spinal cord and cauda equina, presenting as progressive lower extremity weakness. Sensory impairments are rare and bowel/bladder function is usually normal with the lower motor neuron syndrome. Weakness may progress very slowly, allowing patients to remain ambulatory for quite some time after initial presentation. Spinal cord hemorrhage is another late complication of RT. Radiation to the spine may lead to the development of vascular malformations such as telangiectasias and cavernomas, which may rupture and bleed. Patients present with acute onset of lower extremity weakness and back pain.
Safety in Exercise with Brain and Spinal Cord Tumors
The American College of Sports Medicine (ACSM) recommends all cancer patients participate in moderate to vigorous exercise as their condition allows. The ACSM also advises that a patient’s physical activity level and current medical status be assessed at the time of cancer diagnosis, during treatment, and at the time of completion. Assessing the patient at each interval will help to individualize exercise programs with the goal of improving overall health and function. Patients participating in a supervised exercise program have shown the most significant functional improvements and for this reason it is necessary for the treating team to be aware of the patient’s diagnosis, treatments, and both short- and long-term complications of the disease itself or the required treatment.
Participation in a rehabilitation program for patients with brain or spinal cord tumors has shown to improve functional outcomes. However, at present time there are no specific guidelines for the safety of exercise in patients with brain or spine cancer. Therefore, it is recommended the guidelines established by the ACSM for exercise in patients with cancer be followed. The ACSM recommends all cancer patients should avoid periods of inactivity, even when undergoing treatment, and should engage in regular aerobic, resistive, and flexibility exercises. The ACSM realizes modifications will need to be made for certain individuals, but emphasizes the goal of remaining as physically active as their condition allows. A recent study found there to be no adverse outcomes when patients with high-grade glioma participated in a daily exercise program consisting of walking and resistive and balance exercises.
While outlining a supervision of an individual’s rehabilitation and exercise program, the following complications of medical conditions should be taken into consideration. Modifications to individualized programs should be made as needed in order to maintain patient safety.
Cardiac and Pulmonary Safety Considerations
Cancer survivors commonly receive cardiotoxic chemotherapeutic agents, which when combined with RT may injure cardiac muscle and coronary arteries. Awareness of cardiac compromise is warranted in order to customize the patients’ rehabilitation program. Cancer survivors undergoing rehabilitation should be monitored for symptoms of chest pain, dizziness fatigue, leg cramps, and claudication. Vital signs such as pulse oximetry and respiratory rate should be closely monitored during exercise. Cancer survivors may experience pulmonary complications both from the cancer itself (primary and metastatic) and exposure of lungs to RT leading to pneumonitis and pulmonary fibrosis. In addition, cancer survivors are at high risk for PE and aspiration pneumonia. A high index of suspicion for dysphagia is necessary for patients with brain tumors to minimize risk of pneumonia. Cancer survivors may experience dyspnea and decreased exercise tolerance in the rehabilitation setting. It is necessary to monitor for symptoms such as shortness of breath and dizziness as well as assess oxygen saturation prior and during exercise.
Cytopenia While on Chemotherapy
Introduction
Chemotherapy is an important part of the treatment regimen in most individuals with cancer. Temozolomide, bevacizumab, and irinotecan are commonly used in the treatment of primary brain tumors with good response rates. Spinal cord and brain tumors resulting from metastases are generally treated with chemotherapy agents for their primary cancers. The most common cancers that metastasize to the brain are from lung, breast, melanoma, renal, and colorectal, with the incidence of metastases to the brain increasing as the survivorship of the abovementioned malignancies continue to improve. All chemotherapeutic agents have short- and long-term adverse effects that can have effects on safety during rehabilitation.
Anemia
Anemia is a common complication experienced by patients undergoing chemotherapy as a part of their oncologic treatment regimen. Patients may commonly report symptoms of fatigue and dyspnea on exertion, both of which cannot only limit their activity tolerance but also affect their quality of life over time. The prevalence of anemia in adults with cancer is estimated to be as high as 39%, with numbers nearing 100% in those undergoing active chemotherapy. Studies have shown greater hemoglobin concentrations are linked to better physical and functional well-being, with direct correlation between uncorrected anemia and functional disability, even increased mortality. Most individuals will tolerate exercise despite having anemia, with no signs of tachycardia or dyspnea on exertion even with hemoglobin concentrations as low as 8 g/dL. Cardiopulmonary functional capacity and muscular performance can be influenced by anemia, with greater functional capacity decline occurring when there is an acute drop in hemoglobin concentration rather than a gradual drop. Therefore, monitoring the signs and symptoms of chemotherapy-induced anemia, as well as how rapidly the decline occurs in hemoglobin and hematocrit, is paramount to improving and maintaining a patient’s physical and functional performance, as well as increasing cancer treatment tolerance.
Neutropenia
Chemotherapy-induced neutropenia is a serious adverse effect experienced by some cancer patients, and it can be associated with severe localized and systemic infections and prolonged hospitalizations. The risk of serious infection increases with increasing grade and duration of neutropenia, especially neutropenia greater than 7 days’ duration with neutropenic fever being considered an oncologic emergency. It is important to note that these patients are most susceptible to infections that result from their own normal flora, especially those present in the gastrointestinal tract, than nosocomial infections. Therefore, it has been found that increased barrier protection, such as the use of gowns, gloves, and masks, is not useful in the prevention of infection; beneficial recommendations include proper hand hygiene among caregivers, grouping infected patients together, placing neutropenic patients in private rooms, and assigning caregivers to neutropenic patients so that their exposure to other infected patients is low. In an inpatient rehabilitation setting, safety consideration should be given as to whether neutropenic patients should perform their therapies in a commonly used space or their own private rooms.
Thrombocytopenia
Thrombocytopenia is the most common hematologic toxicity seen as a result of chemotherapy use and can lead to serious consequences, such as bleeding complications. It usually results from cumulative toxicity that is dose dependent and is observed 6–14 days after a treatment cycle. The predominant cause of low platelet counts secondary to chemotherapy results from decreased platelet production. Temozolomide was found to be associated with the development of thrombocytopenia in 15%–20% of those undergoing treatment, with thrombocytopenia often being prolonged and possibly irreversible. Aside from the changes to the chemotherapy regimen, there are only a few treatment options available for chemotherapy-induced thrombocytopenia. However, physical exercise regimens have been well tolerated in individuals undergoing active chemotherapy cycles with no signs of hemorrhage with platelets less than 10,000 cells/mm 3 . Therefore, the general recommendations are to limit activities to walking and ADL with platelet counts <20,000 cells/uL; light exercise with close symptom monitoring with platelet counts >20,000 cells/uL; and moderate exercise with light resistive exercises with platelet counts >30,000 cells/uL. While careful consideration should be taken when evaluating thrombocytopenic patients in the rehabilitation setting, physical activities do not necessarily need to be held as long as the patient is able to tolerate the exertion.
Chemotherapy-Induced Peripheral Neuropathy
The peripheral nervous system is vulnerable to the neurotoxic effects of chemotherapy agents. Chemotherapy-induced peripheral neuropathy (CIPN) is frequently a dose-limiting adverse effect and can produce a long-standing negative impact on a patient’s mobility. The nerve fibers and dorsal root ganglia of the primary sensory neurons are equally at risk of being affected, and the development of neuropathy is due to total cumulative dose and dose intensity. Neuropathic changes are most often seen with platinum-based, taxane, and vinca alkaloid agents, and usually occurs in a stocking/glove distribution with sensory disturbances more common than motor disturbances. Patients will often present with decreased vibratory sensation in the toes, as well as a loss of Achilles deep tendon reflexes, with associated numbness, tingling, and paresthesia in the fingers and toes, although some patients may also experience a loss of pain and temperature sensation with taxanes.
CIPN occurs during chemotherapy cycles, with symptoms decreasing after completion of treatment. However, a number of patients can continue to have altered sensation even after the cessation of the chemotherapy regimen. In a secondary data analysis of 512 female cancer survivors, 47% still reported symptoms of CIPN an average of 6 years after completion of chemotherapy, with significantly more disability and 1.8 times the risk of falls, effectively showing that those with CIPN continue to have proprioceptive and balance deficits that put them at risk for further functional disability. Therefore, proper management includes baseline assessments of strength, sensation, balance, proprioception, and gait should be done prior to initiating chemotherapy, with continued screening for changes in the aforementioned clinical measures throughout the duration of the chemotherapy cycles , . Patients who have been on a chemotherapy agent known to cause CIPN should be properly monitored for fall risk throughout their rehabilitation course.
Cognitive Impairments
Changes in cognition secondary to chemotherapy, referred to colloquially as “chemo brain,” can occur in a number of patients. However, cognitive impairment in this population can be multifactorial, as many of these patients not only have a brain tumor located in areas of the brain associated with memory, calculation, and executive functioning but also are on a number of medications that can cause confusion, lethargy, attention, and concentration deficits. Moreover, cognitive impairment can have serious ramifications on quality of life, with reduction in learning and memory the most commonly affected. There is strong evidence that chemotherapy in cancer patients can cause a decline in short-term cognitive ability, but data regarding long-term effects still remains unclear. For those who continue to have cognitive impairments despite stopping chemotherapy, an increase in physical activity and exercise has been shown to improve cognitive function. Yoga, meditation, and Tai Chi have been indirectly linked to improving cognitive function by decreasing stress and fatigue. Pharmacologically, methylphenidate in low doses has been found to be effective in glioblastoma multiforme patients with improvement seen in memory, reasoning, and verbal fluency. Donepezil, ginkgo biloba, hyperbaric oxygen, bevacizumab, and indomethacin have been studied for use in brain tumor populations as well, although the findings have been limited.
Side Effects of Seizures and Anticonvulsants
Seizures are a common presenting symptom of brain tumors, occurring in 20%–80% of patients, especially those with low-grade tumors. While some may have a seizure that leads to the diagnosis of their brain tumor, others develop seizures later in the progression of the disease. Seizures can occur with both primary brain tumors and brain metastases. Tumors involving cortical structures of the brain are more likely to produce seizures, especially those in the temporal and primary sensorimotor cortices.
Traditional first-line agents for seizure management included typical anticonvulsants, such as phenytoin, carbamazepine, and valproic acid for several years. Although these medications have great efficacy for seizure control, they have the common adverse effect of cytochrome P450 enzyme induction, which leads to several drug-drug interactions, especially with chemotherapy agents and glucocorticoids. This can reduce the effective serum concentrations of chemotherapy medications; it can also reduce the efficacy of glucocorticoids in the maintenance of vasogenic edema. In turn, chemotherapy metabolism by the same hepatic enzymes can cause reduction in the serum concentrations of the anticonvulsant agents, thereby increasing a patient’s risk of having seizure activity, which can have devastating neurologic consequences. Typical anticonvulsants also have a higher risk of causing morbilliform drug rashes when taken in conjunction with RT, with a small percentage of patients developing life-threatening reactions such as Stevens-Johnson syndrome.
There have been some reports that levetiracetam can cause increased agitation or negative behavioral changes, but this was seen more often in those with baseline psychologic and behavioral derangements. It is an otherwise well-tolerated anticonvulsant. Newer anticonvulsants, such as levetiracetam and lacosamide, have shown to be equally efficacious to the traditionally used medications, with less adverse effects experienced by the patient while undergoing treatment.
Side Effects of Steroids
Glucocorticoids are an essential part of the treatment of central nervous system tumors. They are used in about 70%–100% of patients with primary central nervous system tumors and metastases and can provide transient relief of neurologic symptoms by decreasing the vasogenic edema that causes increased intracranial pressure or spinal cord compression; a marked improvement is often seen within 24 hours of administration. Glucocorticoids have also been used in neuro-oncology because it can provide short-term relief of chemotherapy-induced nausea and vomiting. Dexamethasone, a fluorinated steroid, is the most commonly used of these medications because of its long half-life and low mineralocorticoid effects; it is known to be beneficial in the management of mass effect secondary to tumor-associated edema and may even protect against the development of acute encephalopathic changes related to RT. However, they are not without their own adverse effects ( Table 2.1 ).