Fig. 7.1
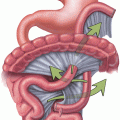
Roux-en-Y gastric bypass
History of Gastric Bypass
The origins of the gastric bypass operation date back to the 1960s and the contributions of Dr. Mason. While searching for a weight loss operation without the detrimental side effects often seen with the jejuno-ileal bypass, he noted that patients with peptic ulcer disease who had undergone a partial gastrectomy with Billroth II gastrojejunostomy subsequently experienced weight loss and had difficulty regaining that lost weight. Based on these findings, Mason described the original gastric bypass in 1967 using a technique that divided the stomach and anastomosed a jejunal loop to the proximal gastric pouch [7, 8]. His original description emphasized a small pouch as well as a small diameter anastomosis to delay gastric emptying and enhance satiety [8]. Over the ensuing several decades, there have been a variety of technical modifications, including attempted calibration and standardization of gastric pouch size [9] and the replacement of the loop gastrojejunostomy with a Roux-en-Y configuration [10]. In 1995, Wittgrove reported the first laparoscopic RNYGB [11]. Since this time, data from the Nationwide Inpatient Sample from 2003 through 2008 have documented an increase of laparoscopic bariatric operations from 20 % in 2003 to 90 % in 2008 [12]. Minimally invasive techniques now account for the vast majority of bariatric procedures performed today and should be considered the standard of care in primary procedures.
Operative and Technical Details
Despite the evolution and almost universal adoption to a laparoscopic technique, there still remain significant technical variations in the way a RNYGBP can be performed. Owing to its complexity and the need for a high degree of laparoscopic technical skills, the relative risk of complications has been reported to diminish following a 500-procedure learning curve [13]. The number of surgeries performed, or experience of the operating surgeon, and the standardization of the laparoscopic technique have been cited as main factors contributing to improved rates of postoperative complications, mortality, and decrease in conversion to open rates [13]. The currently adopted surgical principles, as described by Pories et al. include the exclusion of a majority of the stomach (~ 90 %) except for a 15–30-ml pouch created around the gastroesophageal junction, and transection of the jejunum 30–60 cm from the ligament of Treitz [14]. The distal jejunal segment (Roux limb) is then anastomosed to the gastric pouch and the proximal jejunal segment (biliopancreatic limb) connected distally as a jejunojejunostomy approximately 75–150 cm from the ligament of Treitz, providing secretory drainage of the bypassed stomach, duodenum, and proximal jejunum (common channel limb). The Roux limb, or alimentary limb, carries ingested food and is delineated between the gastrojejunostomy and the jejunojejunostomy. The Roux limb, the excluded or remnant stomach, duodenum, and proximal jejunum represent the bypassed section of the RNYGB [3, 14]. Variations in operative techniques other than gastric pouch size and Roux limb length have included various positionings of the Roux limb and gastrojejunal anastomosis in relation to other body structures. Combinations have included antecolic (above colon) versus retrocolic (behind colon) placement of the Roux limb and antegastric (above stomach) versus retrogastric (behind stomach) creation of the gastrojejunostomy. Techniques for construction of the gastrojejunostomy have included: one layer versus two-layered anastomosis, stapled versus hand-sewn anastomosis or combinations of techniques. Other major variations include routine versus selective closure of mesenteric defects [15, 16].
Outcomes
Multiple studies have demonstrated that among morbidly obese patients, compared with nonsurgical control patients, the use of RNYGB has been associated with higher rates of diabetes remission and lower risk of cardiovascular and other negative health outcomes over long-term follow up [17–20]. In contrast, the cardiovascular and metabolic status of obese control participants generally worsens over time, despite aggressive medical management. When comparing RNYGB with lifestyle and intensive medical management, achievement of primary end points including hemoglobin A1C level, low-density lipoprotein cholesterol levels, systolic blood pressure, and the number of medications needed to manage these comorbidities, the literature has consistently supported gastric bypass, although at the potential expense of increased nutritional deficiencies and potential surgical complications [21].
Weight Loss
Several studies have evaluated the degree of weight loss following gastric bypass, and the durability of the lost weight . A meta-analytic review of the recent published surgical experience found an excess weight loss of 68.2 % (61.5–74.8 %) for RNYGB [22]. A randomized series reported by Nguyen et al. examined 111 laparoscopic RNYGB during a 4-year follow-up period. The authors reported 68.4 % excess weight loss (EWL) for RNYGB with a mean body mass index (BMI) decrease of 15 kg/m2 [23]. In a smaller series, Spivak et. al. reported an EWL of 58.6 % at 7 years follow-up [24]. However, after an initial 4 years of progressive weight loss,the authors reported that most patients experience a certain weight regain during a long-term follow-up period [24]. The prospective, controlled Swedish Obese Subjects study reported a 7 % mean weight regain among patients after gastric bypass surgery from 2 years to 10 years [20]. A more recent study described similar results, with a weight regain of approximately 7 % at 2 years following gastric bypass surgery [17]. Overall, it has been found that for the majority the weight loss is durable and weight regain is modest. It is unclear, at present, if the weight regain represents the normal weight trajectory of the patient’s weight over time or a consequence of physiological or behavioral adaptations to the surgical changes performed .
Diabetes Mellitus
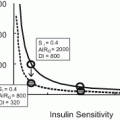
Insulin resistance constitutes a central pathophysiology of type II diabetes mellitus (DM) in the obese. Long-standing remission of DM has been reported to occur in up to 83 % of individuals undergoing RNYGB [3, 18, 25, 26]. In the Surgical Treatment and Medications Potentially Eradicate Diabetes Efficiently (STAMPEDE) trial, intensive medical therapy was compared to surgical treatment (gastric bypass or sleeve gastrectomy) as a means of improving glycemic control. The proportion of patients with the primary end point was 12 % in the medical-therapy group versus 42 % in the gastric bypass group (P = 0.002) [27]. The mechanisms by which gastric bypass results in prolonged improvement in glycemic control have been the subject of a great deal of scientific study. Weight loss, reduced oral intake, altered enteric hormonal secretion (glucagon-like peptide-1, glucose-dependent insulinotropic peptide, ghrelin), and changes to the gastrointestinal tract microflora, all having been proposed as playing a part in this phenomenon [28–34]. Caloric restriction in obese patients without surgery improves insulin sensitivity and glucose homeostasis significantly before any evidence of weight loss is seen [35]. Similarly, all types of bariatric surgery include an element of caloric restriction, which may be the common cause for the associated improvements in insulin sensitivity independent of anatomic changes [3, 36]. However, the immediate effects in glycemic control often precede substantial weight loss typically associated with insulin sensitivity [3]. These observations are consistent with findings by Schauer et al. where reductions in the use of diabetes medications occurred before the achievement of maximal weight loss, suggesting that caloric restriction alone is unable to explain the complex effects RNYGB has on DM [21, 27, 37–39].
Despite the expectation that DM may undergo remission, or at least improve following RNYGB, there is a relative paucity of data attempting to identify patient-specific predictive factors. Hayes et al. used 13 preoperative parameters and a variety of statistical techniques to create mathematical models able to correctly identify which patients would experience remission of DM at 12 months follow-up in 82.7–87.4 % of cases[40]. The two strongest predictors of DM resolution were low HbA1c and the nature and level of preoperative DM control [25, 40, 41]. Other predictive factors that have been proposed for long-term DM remission have included gender (males more likely than women), younger age, and percentage of postoperative EWL [42, 43].
Dyslipidemia
Hyperlipidemia plays a major role in the excess morbidity and premature mortality of the morbidly obese. Improvements in coronary risk factors, including hyperlipidemia, after gastric bypass have been predicted to lower the 10-year risk of ischemic heart disease events by approximately 50 % [44]. Nguyen et al. showed a significant positive effect on atherogenic lipids with improvement of favorable lipoprotein levels after RNYGB that were sustained for the duration of the study [45]. Improvement in lipid profiles was observed as early as 3 months postoperatively and sustained at 2 years follow-up. One year after gastric bypass, mean total cholesterol levels decreased by 16 %; triglyceride levels decreased by 63 %; low-density lipoprotein cholesterol levels decreased by 31 %; very-low-density lipoprotein cholesterol decreased by 74 %; total cholesterol/high-density lipoprotein cholesterol risk ratio decreased by 60 %, and high-density lipoprotein cholesterol levels increased by 39 %. Also, within 1 year, 82 % of patients requiring lipid-lowering medications preoperatively were able to discontinue their medications [45].
Operative Risk Stratification
A large meta-analysis of over 29,000 patients reported mortality of gastric bypass to be 0.5 %.(4) The obesity surgery mortality risk score (OS-MRS) developed by a single institution experience of 2075 patients and subsequently validated used a 90-day all-cause death rate, not just hospitalization, and confirmed a high-risk predictive score [46, 47]. The OS-MRS assigns 1 point to each of 5 preoperative variables , including body mass index > = 50 kg/m2, male gender, hypertension, known risk factors for pulmonary embolism (previous thromboembolism, preoperative vena cava filter, hypoventilation, pulmonary hypertension), and age > = 45 years [48, 49]. Overall mortality for the validation cohort was 0.7 %, consistent with published standards, and represents an average mortality risk among all patient subgroups. Although the highest-risk group class based on the OS-MRS score experienced a 12-fold increase in mortality compared with the lowest-risk group patients, the authors emphasized that the increased risk should not preclude consideration for surgical treatment, as these high-risk patients represent those with a high-risk of early death from their associated comorbidities of obesity.
Postoperative Adverse Outcomes
Anastomotic Leak
Unexplained tachycardia, dyspnea, oliguria, and hypoxia may be warning signs of a leak; however, initial signs and symptoms may be minimal in the morbidly obese. An expected leak rate has been reported to be 0.5 % for laparoscopic RNYGB and remains the most common cause of death after bariatric surgery, followed by pulmonary embolism [50]. A high index of suspicion is warranted and emergent re-exploration may be indicated in patients with unexplained septic physiology.
The primary goal is to obtain adequate drainage of the leak (thereby creating a controlled fistula), bowel rest, broad-spectrum antibiotics, and nutritional support either parenterally or by distal enteral feeding through a gastrostomy tube placed in the excluded stomach to promote healing of the leak. Imaging studies that may assist in diagnosing a leak include an upper gastrointestinal (UGI) study or computerized tomography (CT) scan with oral contrast.
Internal Hernia/Small Bowel Obstruction
Defects in the mesentery are created when transecting the small intestine and reconstructing continuity in a Roux-en-Y configuration. Several potential spaces allow for small bowel herniation with the possibility for strangulation and necrosis of a large intestinal segment. Despite this, routine closure of mesentery is controversial. According to the literature, nonclosure of the defects with the laparoscopic RNYGB technique (antegastric, antecolic), results in an internal hernia rate of 6.9 % [51]. Higa recently demonstrated that after systematic closure of both the mesenteric defect and the Petersen defect with nonresorbable material, the occurrence of internal hernia after transmesocolic, antegastric laparoscopic RNYGB decreased from 16 % to < 1 % [52]. Conversely, Rosenthal’s group reported that the chances of developing an internal hernia are low and do not justify the additional time and possible complications associated with closure of defects [53].
Any patient having undergone RNYGB presenting with abdominal pain and signs or symptoms consistent with bowel obstruction must be considered to have an internal hernia until proven otherwise. The consequence of a delayed diagnosis of an internal hernia is infarction of an extensive portion of small bowel. Persistent abdominal pain in the setting of a negative radiologic study does not rule out the possibility of internal herniation. Diagnostic laparoscopy may serve as the most expeditious diagnostic and potentially therapeutic modality for post-gastric bypass patients with abdominal pain, either definitively “ruling-out” or “ruling-in” an internal hernia while allowing for immediate repair.
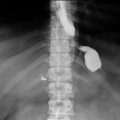
In the immediate postoperative period, obstruction distal to the jejunojejunostomy can cause acute distention of the gastric remnant, which is a surgical emergency. Patients may present with severe bloating and persistent hiccups with a plain abdominal X-ray demonstrating dilatation of the remnant stomach.
Marginal Ulceration
The incidence of marginal ulceration at the gastrojejunal anastomosis ranges from 0.6 to 16 % [54–57]. This number increases to 53.3 % when the complication of gastrogastric fistula is also present [58]. Several factors, including gastric acid, foreign body reaction, exogenous substances such as alcohol, nicotine, and nonsteroidal anti-inflammatory drugs, and Helicobacter pylori infection, have been implicated as potential etiologies causing mucosal ischemia and subsequent marginal ulcer formation [57, 59]. The majority (> 90 %) of patients diagnosed with a marginal ulcer will experience complete resolution of symptoms with proton pump inhibitors and sucralfate.
Anastomotic Stricture
Factors associated with the formation of strictures include operative technique, tension and ischemia at the anastomosis, and ulcer formation [60]. Gradual onset of intolerance to solids or liquids, epigastric pain, and vomiting may signify the development of a stricture. Stricturing at the gastrojejunostomy presents differently than a stricture distally at the jejunojenostomy, as the biliopancreatic limb would contribute bile reflux. A UGI study may assist in diagnosis, however, endoscopic evaluation may be both diagnostic and therapeutic. Dilatation is generally recommended if the gastrojejunostomy anastomosis stoma diameter is less than 15 mm, with serial dilations sometimes required. After 3 balloon treatments, however, surgical revision is generally recommended. Jejunojejunostomy anastomotic narrowing more often requires operative revision, as they are less amenable to endoscopic intervention. The efficacy of newer endoscopic techniques, such as stenting, is yet to be validated.
Gastrogastric Fistula
An abnormal reconnection between the gastric pouch and remnant stomach occurs in approximately 1.2 % of patients following RNYGB [58]. Risk factors for developing a gastrogastric fistula include marginal ulceration and postoperative leak . Symptoms are vague but include nausea, vomiting, and epigastric pain in the majority of patients, as well as insufficient weight loss or weight regain in a minority of patients. The most sensitive test for diagnosing a gastrogastric fistula is an UGI study. Medical treatment may be attempted initially, consisting of acid suppression therapy, particularly if associated with a concomitant marginal ulcer. However, persistent pain, weight regain, and ulceration refractory to optimal medical treatment requires revisional surgery with ligation of the fistula [58].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree