© Springer Science+Business Media New York 2016
Marina Kurian, Bruce M. Wolfe and Sayeed Ikramuddin (eds.)Metabolic Syndrome and Diabetes10.1007/978-1-4939-3220-7_1313. Roux-en-Y Gastric Bypass
(1)
Bon Secours Maryview Medical Center, Portsmouth, VA, USA
(2)
Department of Surgery, Virginia Commonwealth University, Richmond, VA, USA
Keywords
Metabolic surgerySurgical treatmentDiabetesGastric bypassRoux-en-Y limb (RYGBP)Bariatric surgeryAnastomotic leaksStomal stenosisCholelithiasisDumping syndrome13.1 History
Gastric bypass (GBP) was developed and described by Drs. Mason and Ito in 1967 [1]. The procedure was created on the basis of the weight loss initially observed in patients undergoing partial gastrectomy for treatment of ulcers. Over the past several decades, the gastric bypass has been modified into its current most performed form, using a Roux-en-Y limb (RYGBP) . According to the American Society for Metabolic and Bariatric Surgery (ASMBS), 179,000 bariatric surgeries were performed in 2013, of which 34.2 % were RYGBP.
13.2 Procedure
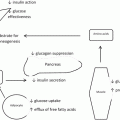
Gastric bypass procedure s typically involve creating a proximal gastric pouch reservoir with a surgical anastomosis between the pouch and the small intestine. Thus oral intake “bypasses” the remaining stomach, duodenum and some variable portion of the small intestine, depending upon how much intestinal bypass is performed. The procedure is most commonly accepted with creation of a Roux-en-Y limb of jejunum for anastomosis to the proximal gastric pouch; however, techniques for creating a loop gastrojejunostomy are also done in some parts of the world. Gastric pouch creation is accomplished with surgical stapling devices, the more modern iterations of these staplers (and those used uniformly in laparoscopic gastric bypass) typically staple and transect the tissue between the rows of staples, creating what has been called a “divided” gastric bypass. In contrast, when performed via “open” surgical access, many surgeons did not transect the gastric pouch from the distal gastric remnant, creating a gastric bypass with “in continuity” gastric stapling. The gastric pouch volume recommended is small, on the order of 25–30 cm3 or less, but there is controversy as to whether pouch volume is a critical component of the procedure from the perspective of the metabolic effects of the surgery which will be described in detail below. It is likely that a small gastric pouch volume contributes primarily to the restrictive effect of the procedure, specifically influencing the degree of weight loss and, perhaps, the risk of weight regain following the procedure, although these issues are also controversial and not well proven in the surgical scientific literature.
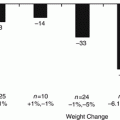
Gastric bypass is often referred to as the “gold standard” for bariatric surgical procedures, in part because the initial weight loss induced by the surgical procedure is somewhat greater and overall less variable than other commonly performed procedures like the adjustable gastric band and the sleeve gastrectomy. The average morbidly obese patient undergoing gastric bypass surgery will typically lose between 60 and 75 % of their calculated excess body weight, and total excess weight is determined by comparison of preoperative body weight to Metropolitan Life tables, which provide information regarding Ideal Body Weight. In contrast, the typical excess weight loss following the restrictive sleeve gastrectomy procedure is in the range of 50–60 % of excess, while the adjustable gastric band procedure typically results in excess weight loss below 50 % of excess .
13.3 Post op Management and Complications
The current overall mortality rate for bariatric surgery, according to the American Society for Metabolic and Bariatric Surgery, is approximately 0.1 %, with the overall possibility of major complications 4.3 %. Complications of RYGBP include anastomotic leaks, internal hernia, stomal stenosis, marginal ulcers, cholelithiasis, dumping syndrome, changes in nutritional absorption, along with any other issues that may occur as a consequence of surgery (i.e., wound infections, atelectasis, pneumonia, deep vein thrombosis, etc.).
Patients who have undergone laparoscopic RYGBP are selectively examined postoperatively with a double contrast (water-soluble contrast and barium sulfate) upper gastrointestinal series (UGI) . The purpose of the study is to evaluate for the presence of an anastomotic leak. If no evidence of leak is apparent, patients are initiated on a clear liquid diet, with a rate often no greater than 60 mL/h initially. After the patient tolerates the liquid diet for 24 h, some surgeons advance the diet to pureed food (with no added sugar). The pureed diet is continued for 1 month postoperatively, after which regular food may be gradually incorporated. Patients should be instructed to abstain from any foods and liquids that are high in simple carbohydrates and to avoid sugars and sweets.
13.3.1 Anastomotic Leaks
The two sites of anastomoses (gastrojejunostomy and jejunojejunostomy) may serve as a potential source of complications. Anastomotic leak risks range from 0.8 to 6 %, with most generally occurring within 1 week of surgery, but may occur up to 1 month postoperatively. The patient presentation may include signs such as low-grade fevers, respiratory distress and/or tachycardia (greater than 120 beats per minute). If a leak is suspected clinically, even if imaging is negative, emergent surgical exploration is indicated, and is often done laparoscopically. To test for a possible leak intraoperatively, methylene blue or endoscopy may be utilized. For evaluation with the dye test, methylene blue with saline is injected via a nasogastric tube while a bowel clamp is placed distal to the gastrojejunostomy. To evaluate endoscopically, saline is used to submerge the gastrojejunostomy while the roux-limb is clamped and the pouch is insufflated via the endoscope. Air bubbles present in the saline around the pouch may indicate the presence of a leak. Treatment for an anastomotic leak includes drainage, broad-spectrum antibiotic coverage and identification/repair of the defect when feasible. A gastric tube may be placed for feeding or intravenous feedings may be used.
13.3.2 Stomal Stenosis
Stomal stenosis is a stricture that may form at the gastrojejunal anastomosis. Incidence of stomal stenosis after RGYBP is reported to be between 3.1 and 15.7 %. The etiology of stenosis may be related to the method used for anastomosis, with the greatest risk seen in the use of a 21 mm circular staple, followed in order by a 25 mm circular stapler, linear stapler, and hand-sewn anastomosis [2]. Patients present 1–2 months postoperatively with nausea, vomiting, dysphagia, and/or gastroesophageal reflux, with ultimate progression of intolerance of oral intake. Diagnosis is made by endoscopy or upper GI series. Treatment is endoscopic dilation as the preferred and less invasive option. A therapeutic endoscope is inserted through the gastrojejunostomy along with a balloon dilator which is pneumatically insufflated based on the stoma size. The complication rate is about 3 %, and repeated dilation may be required for some patients. Surgical revision is reserved for patients who have persistent stenosis despite repeated endoscopic dilations .
13.3.3 Marginal Ulcers
Marginal ulcers are mucosal erosions that occur at the gastrojejunal anastomosis, most commonly on the jejunal side. The ulcers typically occur when the gastric remnant is stapled but not divided. The incidence is reported to be between 0.6 and 16 %. Possible etiologies of marginal ulcers include nonsteroidal anti-inflammatory (NSAID) drug use, Helicobacter pylori (H. pylori) infection, smoking, poor tissue perfusion secondary to excess tension or ischemia at the anastomosis, increased exposure to acid in the gastric pouch due to gastrogastric fistula formation, and presence of foreign material (suture or staples). Patient presentation includes nausea, pain, with possible bleeding and/or perforation. Diagnosis is made by upper endoscopy. Initial management includes medical treatment with gastric acid suppression (proton pump inhibitors) with or without sucralfate. Patients should be strongly encouraged to stop NSAID use and smoking. Patients with H. pylori colonization should be treated with triple therapy (proton pump inhibitor, clarithromycin and amoxicillin).
13.3.4 Cholelithiasis
Cholelithiasis may develop in up to 38 % of patients undergoing gastric bypass surgery, and up to 41 % of these patients become symptomatic. Rapid loss of weight can contribute to the formation of gallstones by increasing the lithogenicity of bile. This risk can be significantly reduced to 2 % when patients are postoperatively treated with a 6 month course of ursodeoxycholic acid [3]. Unfortunately compliance with ursodeoxycholic acid prophylaxis may be poor due to gastrointestinal side effects of the medication. Symptomatic patients should be evaluated preoperatively or intraoperatively for cholelithiasis and should have a cholecystectomy performed at the time of gastric bypass if gallstones are present. Asymptomatic patients may be managed expectantly as a minority will come to cholecystectomy during follow-up.
13.3.5 Dumping Syndrome
Dumping syndrome is a well-known physiologic phenomenon associated with gastric bypass, as it occurs in procedures that involve partial or complete gastrectomy. Dumping typically occurs when patients ingest high levels of simple carbohydrates. Hormones thought to be involved in this mechanism include enteroglucagon, vasoactive intestinal peptide (VIP), peptide YY (PYY), pancreatic polypeptide, and neurotensin. Dumping may be divided into early and late phenomena. Early dumping occurs within 15–30 min of ingestion and is due to rapid gastric emptying. The high osmolality of the food causes rapid fluid shifts from the plasma into the bowel, which leads to hypotension and reflex sympathetic nervous system activation. Patients will present with symptoms of colicky abdominal pain, diarrhea, nausea, and tachycardia. Late dumping occurs 1–3 h after meal ingestion, and presents with symptoms of hypoglycemia (dizziness, fatigue, diaphoresis, weakness, confusion). Rapid gastric emptying leads to a high glucose concentration, which is rapidly absorbed and triggers insulin secretion, leading to the signs and symptoms of hypoglycemia. Sigstad’s scoring system may be used for diagnosing dumping syndrome, where a score greater than seven is suggestive of dumping, and a score less than four suggests other diagnoses. To avoid dumping, patients are advised to avoid foods high in simple carbohydrates, and are encouraged to eat diets with high fiber content, complex carbohydrates, and high protein content. Furthermore, patients are instructed to eat small, frequent meals (up to six per day), and to avoid drinking with meals or in the first 2 h after a meal. If these first line measures fail, somatostatin analogue use may be considered, which are administered subcutaneously three times a day or intramuscularly once every 2–4 weeks. Although dumping is an unpleasant consequence that patients may have to withstand, the condition actually aids the patient in adhering to the prescribed dietary restrictions by acting as negative feedback to the ingestion of sugar [4].
13.3.6 Nutritional Changes
Changes in nutritional needs are important to be considered when managing a post-gastric bypass patient. Patients are advised to obtain a significant amount of their daily calories from protein (50–60 g per day). Daily supplementation also includes 500 μg of vitamin B12, 1200 mg of calcium, and a multivitamin tablet. Women who menstruate are also asked to take 650 mg of ferrous sulfate daily. Serum levels of these supplements should be checked during regular clinic appointments [5].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree