Fig. 17.1
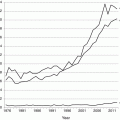
Size distribution of maximal tumor diameter in 4138 adult PTC patients consecutively treated at Mayo during 1935–2014 and demonstrating that one-third of these patients during the eight decades presented with PTM and tumor diameters of 1 cm or less (pT1a)
In 2008 we presented and published [45] our experience of treating 900 pT1a tumors over a 60-year period (1945–2004). These patients were followed up for up to 54 years and on average for 17 postoperative years. At last follow-up, only three patients (0.3%) died of PTC. RRA was administered to 17% of the study group and, when reevaluated for efficacy, was found to be 99% successful in terms of negative neck and whole-body RAI scans. Expected and observed all-causes survival were near identical (p = 0.96). CSM rates at 20 and 40 years were 0.1 and 0.7%.
Of 758 patients without distant spread, undergoing BLR with complete tumor resection, 119 (16%) had RRA administered within 6 postoperative months. RRA did not impact tumor recurrence (TR) at local or distant sites, but postoperative (“recurrent”) neck nodal metastases (NNM) were more frequently found after RRA, when compared to those treated by BLR only. These higher NNM rates were likely explained because node-positive patients were 10 times more likely to have received RRA. Four percent of node-negative PTM patients got RRA, and at 20 years TR rates were 0.6% after BLR and 0% after BLR + RRA (p = 0.79). By contrast, 38% of node-positive cases got RRA, which did not decrease TR at either local (p = 0.8) or distant (p = 0.7) sites. Higher TR rates were seen with either multicentric tumors or patients who were node-positive at presentation. Accordingly, for our final analysis of the efficacy of RRA in PTM in our six-decade cohort, we elected to examine four subsets of patients divided according to the number of foci (unicentric vs. multicentric) and presence or absence of NNM at initial surgery.
With unifocal node-negative PTM, RRA did not decrease the <1% risk of nodal recurrence seen after BLR (p = 0.8). In multifocal node-negative cases, no recurrences at any site were seen in 101 patients, perhaps implying that multicentricity per se in PTM does not impart a higher risk of TR. In unifocal node-positive disease, RRA did not significantly reduce the 11% TR seen after BLR alone (p = 0.2). Finally, in the worst-case scenario of multifocal node-positive PTM, RRA did not in 100 cases significantly decrease the 22% TR rate (all sites) seen after BLR alone. Our 2008 conclusions [45] were that the extent of surgery did not affect TR rates, and RRA did not improve outcome in any subset of patients studied, including those with multicentric tumors or those presenting with NNM at initial surgery.
We recently presented [46] at the 2016 Meeting of the Endocrine Society the results from our experience in managing the 1345 PTM patients shown in Fig. 17.1. Of the 1281 potentially curable cases (no distant metastases and complete surgical resection after BLR), only 165 (13%) had RRA within 6 months of successful BLR. Interestingly, only 1% was ablated in the decade of 1965–1974, but in the decade of 1975–1984, when Mazzaferri’s two initial PTC studies [22, 23] were published, this rate rose by more than 20-fold to 23%. However, in the subsequent three decades up to 2014, the rates of RRA dropped progressively from 20 to 16 during 1985–1994 and 1995–2004, respectively, and, most recently, 11% in the last decade of 2005–2014.
Figure 17.2 illustrates, within the eight-decade PTM cohort, the very significant influence (p < 0.001) of NNM at presentation on subsequent discovery over 40 postoperative years of so-called “recurrent” NNM. In this recent study, we again concluded that PTM patients have normal life expectancy and typically are cured by adequate tumor resection. More than 99% of our PTM patients treated over eight decades were not at risk of either distant spread or mortality from cancer. The 20-year TR rates were only 7%, almost exclusively in regional (neck) nodes. The extent of initial surgery [46] did not affect locoregional recurrence rates (p = 0.8) and, most interestingly, the 30-year TR rates in node-positive cases after lobectomy alone were no different from those seen after BLR or even NT or TT followed by RRA (p = 0.99). Figure 17.3 demonstrates that in node-positive PTM (pT1N1MO) the 30-year TR rate after UL was insignificantly different from the comparable rates seen after either (left panel) BLR + RRA or (right panel) NT or TT followed by RRA within 6 postoperative months.
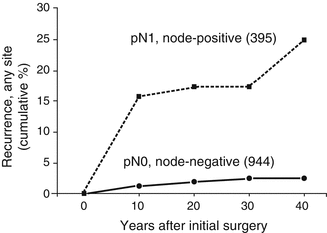
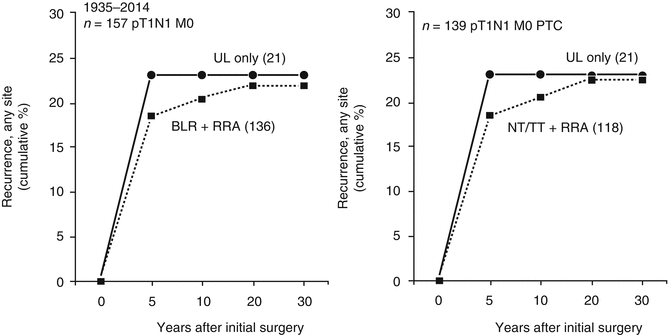

Fig. 17.2
Influence of neck nodal metastases at presentation on cumulative tumor recurrence rates over 40 postoperative years in 1339 patients with localized (M0) papillary microcancers, who had complete primary tumor excision at initial surgery and demonstrating a highly significant (p < 0.001) increase (almost tenfold) in those 395 patients who were node-positive (pN1)

Fig. 17.3
Comparison, within patients with pT1aN1M0 microcarcinoma having potentially curative surgery at Mayo during eight decades (1935–2014), of cumulative tumor recurrence rates over 30 postoperative years and demonstrating that the recurrence risk (at any site, locoregional or distant) after unilateral lobectomy alone was no different (p = NS) from that seen after either bilateral lobar resection (BLR) and RRA (left panel) or near-total or total thyroidectomy (NT/TT) and RRA (right panel)
In 2009, we had reported to the American Thyroid Association at their annual meeting that neither the 2006 ATA Cancer Guidelines [47] nor a more recently published report by Rosario [48] regarding adjuvant therapy in PTC tumors <2 cm diameter provided convincing data regarding a role for RRA in improving postoperative outcome in PTC patients with AJCC pT1 tumors, especially those pT1b tumors with diameters of 11–20 mm. We suggested [49] that if a prospective trial was to be designed to answer this question, it would likely involve “low risk” (e.g., with MACIS scores <6) tumors in patients aged 21 years or older, with surgically curable T1 disease (i.e., neither T4 nor M1) completely resected by initial successful BLR.
In the prospective retrospective study that we presented, [49] our aim was to define outcome in a cohort of 765 adult (aged 21 or older) patients with MACIS <6 pT1M0 PTC treated during the 35 years after the introduction of RRA and before the current era of ultrasound-guided neck nodal biopsies, recombinant human TSH-stimulated thyroglobulin (Tg) testing and near-routine central compartment neck nodal dissection. The 765 patients (545F; 220M) underwent BLR during 1950–1985 for tumors that were completely resected and were neither locally invasive at initial neck exploration nor distant spread at initial presentation.
Median patient age in this 1950–1985 cohort was 45 years (range 21–72), mean tumor size 12 mm (49% <11 mm); 24% were multicentric and 30% node-positive. Mean follow-up was 27 years (longest 55); 23% for >35 years. One hundred and seventy (22%) received RRA. 35-year occurrence rates for CSM, local recurrence (LR), regional nodal metastases (RNM), and distant metastases (DM) were 1.1, 2.2, 5.0, and 1.6%, respectively. Comparable rates for Group A (n = 375 PTM with pT1a tumors <11 mm) were 0.6, 1.8, 4.5, and 0.5%. For Group B (n = 390 pT1b tumors of 11–20 mm diameter), rates were higher at 1.6, 4.3, 5.5, and 2.6%, respectively. RRA’s impact was assessed by comparing survival to each of these four endpoints in patients undergoing BLR alone versus those receiving BLR + RRA within 18 postoperative months. The 35-year CSM rates in Groups A and B were after BLR 0.6 and 1.6% and after BLR + RRA insignificantly different (p > 0.75). Similarly, survival rates to LR, RNM, and DM were no different in ablated patients than after BLR alone in both Groups A (p > 0.07) and B (p > 0.33).
It was our principal conclusion [49] from this study that the results confirmed the excellent prognosis of AJCC pT1 tumors treated by BLR and did not identify a significant reduction in either mortality or recurrence rates in those patients with T1 PTC tumors selected for RRA.
Question 3: If RRA Is Ineffective in Reducing Mortality and Recurrence in PTC Patients with MACIS Scores <6, Should We Be Using RRA Selectively to Treat Only the Minority of Patients with High-Risk PTC, Who Have MACIS Scores of 6 or More?
In an attempt to quantify the influence of RRA on outcome in low-risk PTC after adequate initial surgery, we performed in 2002 [25] and again in 2006 [50] analyses on 1163 MACIS low-risk PTC (scores <6) patients, who had undergone NT or TT during 1970–2000 for tumors confined to the neck that were completely excised at initial neck exploration. 498 (43%) of these patients had RRA within 6 months of the initial surgery. Those who received RRA were more likely to have had NNM at presentation (p < 0.001). Of 636 node-negative patients, 195 (31%) received RRA. However, of 527 node-positive patients, 303 (57%) were ablated.
At 20 postoperative years, the CSM rate for the surgery alone patients was 0.4%, and for the NT/TT and RRA group, it was insignificantly different at 0.6% (p = 0.64). At 20 years, the TR rate was actually significantly higher in the ablated group (14% vs. 9%; p = 0.008), likely reflecting the tendency to more readily ablate node-positive patients. When the patients were divided into node-negative and node-positive groups, there were no statistically significant differences in outcome (CSM and TR) between those having surgery alone and those who also received postoperative RRA. Interestingly, there were no deaths from PTC in the 636 node-negative cases and only two in the node-positive group.
For the node-negative patients, the 20-year TR rates were 3.4% after surgery alone and 4.3% after surgery and RRA (p = 0.80). For the node-positive group, who clearly had much higher TR rates, the CSM rates at 20 years were 1.2% after surgery alone and 0.9% after RRA (p = 0.99). The 20-year TR rates only differed by 0.4%, being 19.5% for surgery alone and 19.9% for surgery and RRA (p = 0.66). Clearly, it was our 2006 conclusion [50] that RRA did not significantly improve the outcome (either CSM or TR) in low-risk (MACIS scores <6) PTC patients previously treated with initial NT or TT with curative intent. This conclusion obviously became a pivotal part of our Mayo policy [51] for managing patients with low-risk PTC published in 2007.
As we prepared for this chapter and were working on updated outcome results from our eight-decade Mayo PTC cohort, we considered it relevant to extend the years of our MACIS <6 cohort a further 14 years to encompass those low-risk PTC patients who were surgically treated definitively, with or without RRA, in the years of 2001–2014. This added a further 911 patients to a new total cohort of 2074 adult MACIS <6 patients, of whom 760 (37%) underwent RRA within 6 months of NT or TT with curative intent. The principal details of the 20-year CSM and TR rates are included in the accompanying Table 17.1.
Table 17.1
Lack of influence of RRA on outcome in 2074 MACIS <6 low-risk PTC patients (without distant metastases) treated at Mayo during 1970–2014 by NT/TT with complete tumor excision
Low risk | 20-Year mortality | 20-Year recurrence | ||
|---|---|---|---|---|
(MACIS < 6) 1970–2014 | NT/TT alone | NT/TT and RRA | NT/TT alone | NT/TT and RRA |
All patients (%) (n = 2074) | 0.3 | 0.7 | 8.7 | 18.2 |
P = 0.09 | P < 0.001 | |||
Node-negative (%) (n = 1159) | 0 | 0.5 | 3.9 | 4.6 |
P = 0.11 | P = 0.34 | |||
Node-positive (%) (n = 915) | 1.0 | 0.9 | 19.1 | 26.3 |
P = 0.53 | P = 0.08 | |||
Figure 17.4 illustrates the differences in TR over 20 postoperative years between ablated and not ablated patients in the entire study cohort of 2074 low-risk PTC patients (left panel), the 1159 node-negative cases (middle panel) and the 915 node-positive patients (right panel). As expected, in the node-positive patients, most recurrences (83%) were situated in regional neck nodes. There were no significant differences between the ablated and the not ablated groups in terms of either local recurrences (p = 0.34) or distant metastases (p = 0.49), generally considered [3, 32] to be postoperative events associated with an increased risk of CSM. Interestingly, the recurrence rate in regional nodes was insignificantly higher (p = 0.05) in the 496 ablated patients, and this was felt to be attributable to significantly higher numbers of NNM found in those patients selected for RRA. The cumulative recurrence rates over 20 postoperative years in the 915 node-positive cases for all three anatomic locations (regional, local, and distant) are illustrated in Fig. 17.5.
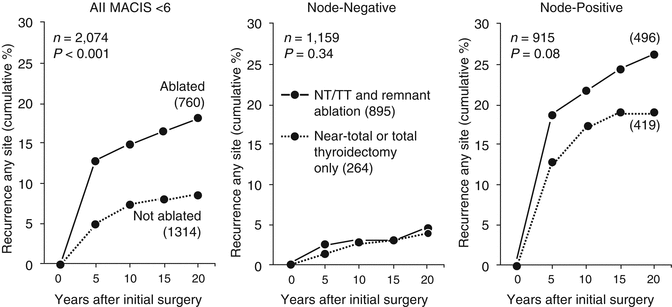
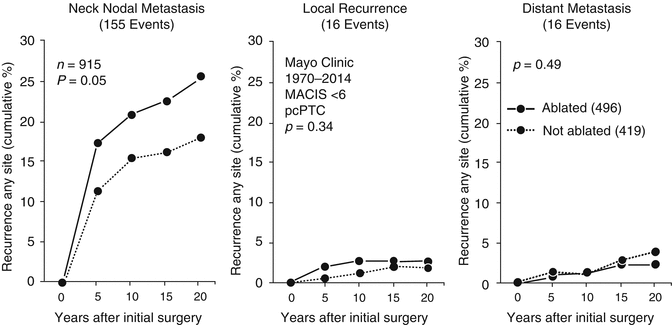

Fig. 17.4
Influence of RRA on tumor recurrence (any site) over 20 postoperative years in 2074 MACIS <6 PTC patients, who had no distant metastases and had undergone complete tumor resection after initial potentially curative surgery with NT/TT during the 35-year period of 1970–2014 (left panel), in 1159 pN0 node-negative patients (middle panel) and in 915 pN1 node-positive patients (right panel). Recurrence rates were higher (p < 0.001) in ablated patients (left panel), but were insignificantly different (p = NS) in either node-negative (middle panel) or node-positive patients (right panel)

Fig. 17.5
Lack of influence of RRA administered within 6 postoperative months, when compared to NT/TT + RRA, in 915 potentially curable MACIS <6 node-positive PTC patients consecutively treated at Mayo during 1970–2014 on rates of cumulative tumor recurrence at regional (left panel), local (middle panel), and distant sites (right panel)
The results of these two studies defining the lack of impact of RRA on outcome in MACIS <6 low-risk PTC have helped convince us that RRA can probably be avoided in, not just the 68% of PTC patients who have pT1 tumors but also the 84% who have MACIS scores <6 and have tumors localized to the neck and having complete primary tumor excision at initial definitive surgery.
Era of Systematic Reviews and Meta-Analyses
That remarkable source of knowledge, Wikipedia, informs us that “conceptually, a meta-analysis uses a statistical approach to combine the results from multiple studies in an effort to increase power (over individual studies), improve estimates of the size of the effect and/or to resolve uncertainty when reports disagree.” Given the rancor expressed in the past 30 years over the vexed question of the efficacy of RRA in low-risk PTC, it is not perhaps surprising that multiple authors have jumped at the chance of “resolving uncertainty when reports disagree.”
Mayo-trained Anna Sawka started this type of study in 2002–2003, while she was working at McMaster University in Hamilton, Ontario. She began by screening 1543 unique references and ended up by studying in great detail those 23 references [27] that “met all inclusion criteria,” one of them from Mayo [25]. Her conclusion [27] from a systematic review of the 1966–2002 literature was that “the effectiveness of RAI ablation decreasing recurrence and possible mortality in low-risk patients with well differentiated thyroid carcinoma, although suspected, cannot be definitively verified by summarizing the current body of observational patient data.” She expressed the opinion [27] that “only a long-term randomized controlled trial may definitely resolve this issue” and concluded that “in the meantime, the decision for RAI ablation must be individualized, based on the risk profile of the patient, as well as patient and physician preference, while balancing the risk and benefit of such therapy.”
Four years later, Sawka published [26] an “updated systematic review” which included data from 20 studies from the original review [27], the original review itself, and seven newer studies from 2002 to 2007. Again, she was unable to identify any long-term randomized controlling trials examining outcomes after RRA; she therefore restricted her review to observational data. Her conclusion in 2007 was that “upon carefully examining the best existing long-term observational evidence, the authors could not confirm a significant, consistent, benefit of RRA in decreasing cause-specific mortality or recurrence in early stage WDTC.” She observed [26] that “in an age of freely available information, patients themselves may have strong opinions about accepting or declining RRA and it is important for physicians to be sensitive to such concerns. The current reality is that decision making about RRA in early stage thyroid carcinoma is a complex, evolving issue and long term higher quality evidence is needed to inform future clinical practice.”
In a more recent systematic analysis of the 1966–2008 peer-reviewed literature, published in 2010, Sacks [52] from Cedars-Sinai reported “that the preponderance of evidence suggests that RAI treatment is not associated with improved survival in patients with low-stage or low-risk DTC. The data concerning recurrence rates following RAI treatment in this group of patients were less conclusive.” On the basis of her analysis, she recommended the adoption of a risk group categorization, based on AJCC TNM staging and the MACIS score, and proposed a management guideline based on a patient’s risk: very low, low, moderate, and high. Her final conclusion was that “a majority of very low-risk and low-risk patients, as well as select cases of patients with moderate risk, do not demonstrate survival or disease-free survival benefit from postoperative RAI treatment, and therefore we recommend against postoperative RAI in these cases.”
Finally, in 2015, an Italian systematic review [53] by a group of investigators from Rome and led by Cooper, the lead author of the 2006 [47] and 2009 [54] ATA Guidelines, concluded that, when compared to earlier meta-analyses [26, 52] of literature until 2007–2008, “our review of the more recent literature (2008–2014) clearly shows no advantage of RRA in low-risk patients, but it was unable to provide conclusive data for or against RRA in preventing disease recurrence in intermediate risk patients.” They recommended from their analysis that “a careful evaluation of tumor pathological features and patient characteristics and preferences should guide RRA decision making.” They expressed hope that the two presently ongoing European prospective randomized trials (the French Estimabl2 study and the British IoN study) will “provide valuable data to inform this issue.” They recommended that “an undetectable serum Tg, especially in a high-sensitivity Tg assay, and negative neck US 6–12 months after surgery should enable many low risk and intermediate risk patients to be categorized as being ‘free of disease’, despite not having undergone RRA,” thereby supporting a position remarkably close to that proposed in the 2007 description of the current Mayo management [51] of patients with low-risk PTC.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree