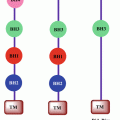
Fig. 6.1
Schematic representation of OPN structure and its receptors. OPN contains binding sites for different integrins at N-terminus and CD44 receptors at C-terminus. It also encompasses calcium-binding site and RGD and thrombin cleavage motifs
6.3 Role of OPN in Stroma-Tumor Interaction
Tumor microenvironment not only consists of cancerous epithelial cells but also various kinds of mesenchymal, vascular, and immune cells. Tumor stroma is composed of fibroblasts, macrophages, MSCs, pericytes, lymphocytes, and endothelial cells. Reciprocal interaction between the tumor and stromal cells is essential for promotion of neoplastic stage to higher grades of tumor (Wels et al. 2008). OPN is known to be involved in regulation of interaction between tumor and stroma by modulating various growth factors and chemokines.
6.3.1 OPN and MSCs in Cancer Progression
MSCs are multipotent progenitor cells capable of proliferation and commonly isolated from bone marrow, amniotic fluid, placenta, cord blood, skeletal muscle, and adipose tissues. Under specific culture conditions, MSCs can be induced to differentiate into different mesodermal and myogenic lineages. Moreover, MSCs can modulate immune response and are therefore used to control autoimmune disorders. Since MSCs can be cultured ex vivo and successfully used for autologous transplantation, they have potential application in regenerative medicine.
Several in vitro as well as in vivo studies have reported that MSCs exhibit tumor-directed migration and stromal incorporation (Loebinger et al. 2009). Studies performed with MSCs and cancer cell lines have demonstrated that MSCs show significant tumor tropism (Kidd et al. 2009). This phenomenon occurs due to tumor-derived cytokines, chemokines, and inflammatory mediators which results in tumor-directed MSC migration and subsequent incorporation in the tumor microenvironment. Tumor-educated MSCs further induced inflammation by recruiting PBMCs (peripheral blood monocytes) that drives tumor growth. This proinflammatory response is mediated by tumor-derived IL-1-β through the FAK/MAPK signaling while TGF-β inhibited this phenotype of MSCs. In the stroma of both primary tumor as well as metastatic sites, MSCs can differentiate into cancer-associated fibroblasts (CAFs) (Koh and Kang 2012). Previously it has been shown that MSCs are capable of enhancing angiogenesis of breast and prostate cancer (Zhang et al. 2013). Studies performed by Spaeth et al. provide evidence that MSCs have the capacity to differentiate into CAFs and induce expression of HGF, IL-6, and VEGF which drive tumor growth and angiogenesis (Spaeth et al. 2009). The presence of MSCs, CAFs, and other dysregulated stromal cells within tumor is associated with poor clinical outcome and enhanced metastatic potential (Quail and Joyce 2013). The mechanism by which tumor cells interact with the stromal cells and how that interaction controls tumor progression are not well defined. McAllister et al. have shown that OPN secreted in instigating primary tumor controls the migration of aggressive cancer cells towards distant nonmalignant tumors through bone marrow cell activation (McAllister et al. 2008). Mi et al. have shown that OPN regulates CCL5-mesenchymal stromal cell-mediated breast cancer metastasis (Mi et al. 2011, Fig. 6.2). Using in vitro and in vivo models, they have shown that tumor-derived OPN binds to integrin and AP-1 transactivation leading to induced CCL5 expression by the MSCs. Upon activation by tumor-derived OPN, MSCs have been shown to metastasize to distant tissues where they differentiate into CAFs and express fibroblast-specific markers: α-SMA, FSP-1, CXCL-12, and tenascin-c. These CAFs have been shown to produce MMP-2 and MMP-9 that mediate cancer progression by remodeling the tissue architecture (Mi et al. 2011).
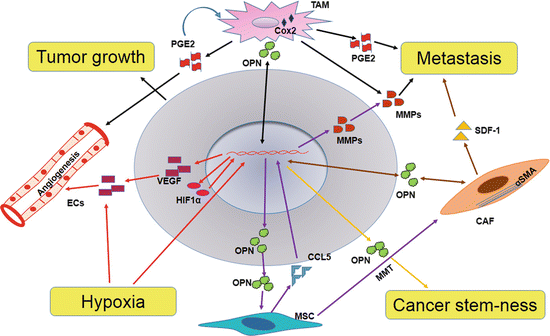

Fig. 6.2
Role of OPN in tumor-stroma interaction. Tumor-derived OPN is involved in the activation of stromal cells in tumor microenvironment. Stroma-derived OPN regulates tumor growth, angiogenesis, and metastasis
6.3.2 OPN and CAFs in Cancer Progression
Major part of tumor stroma consists of fibroblasts and these are imperative for promotion of primary tumors to higher grades and overall cancer spread (Wels et al. 2008). Under normal physiological conditions, fibroblast shows inhibitory role in cell proliferation whereas in tumor context these are activated by the factors secreted by cancerous cells or stromal cells and thereby support the cell proliferation (Wels et al. 2008; Tchou and Conejo-Garcia 2012). Myofibroblast-specific gene expression is the hallmark of CAFs (Cirri and Chiarugi 2011). Several studies have shown that CAFs play multifaceted role in tumor progression and angiogenesis and metastasis by regulating factors linked to proliferation, apoptosis, invasion, and immune evasion (Wels et al. 2008; Tchou and Conejo-Garcia 2012; Cirri and Chiarugi 2011).
Senescent fibroblasts, reminiscent of myofibroblasts, promote the preneoplastic cell growth via OPN (Pazolli et al. 2009). OPN is required for the transactivation of fibroblasts to myofibroblasts by TGF-β and deletion of OPN prevents the activation of fibroblasts (Lenga et al. 2008). Tumor-derived OPN promote breast tumor progression by inducing the transformation of MSC into CAFs with upregulation of CCL5 and myofibroblastic marker α-SMA (Fig. 6.2). Blockade of OPN-mediated CCL5 expression using RGD, a competitive ligand inhibitor of integrin binding, CD44-blocking Ab, or OPN-R3 APT abated tumor growth and metastasis by diminishing CAFs generation (Mi et al. 2011). Recent data have shown that tumor-derived PDGF-CC recruits stromal fibroblasts into tumor and activates to CAFs by engaging with PDGFR-α. In addition, CAFs-dependent melanoma growth is mediated through CAF-derived OPN (Anderberg et al. 2009, Fig. 6.2).
6.3.3 OPN and TAMs in Cancer Progression
Infiltration of mononuclear inflammatory cells proximal to neoplastic tissues is one of the hallmarks of cancer-associated chronic inflammation. Macrophages, dendritic cells, and neutrophils are mainly observed at sites of inflammation. In tumors, cancer cells secrete chemokines which are classified as inflammatory or inducible (Mantovani et al. 2002). Constitutive expression of NF-κB is correlated with increased production of CXCL1 that results in inflammation within the tumor (Yang and Richmond 2001). Studies have shown that infiltration of macrophages in higher frequency is associated with poor prognosis of cancer. Macrophages exhibit two polarized states: M1 phenotype, in which they can invade the site of inflammation by responding to cytokines and chemokines released by neoplastic cells where they adopt the M2 phenotype that helps in tumor progression and metastasis (Mantovani et al. 2002). Recent studies have shown that macrophages overexpress OPN upon co-culturing with breast cancer cells (Solinas et al. 2010). Antagonizing OPN with neutralizing antibody interferes with macrophage function in the tumor microenvironment (Giachelli et al. 1998).
At the site of injury or infection, proinflammatory mediators and growth factors like TNF-α, IL-1, and PDGF increase expression of OPN by activating protein kinase C (Denhardt et al. 2001). In different cancers, OPN-expressing macrophages are more in number near the edge of tumor and in areas of tumor necrosis suggesting their role in invasion and metastasis (Brown et al. 1994). In colorectal cancer, immunohistochemical analyses have shown increased expression of OPN in TAMs (Rao et al. 2013a). Recently Kale et al. have shown that OPN expressing macrophages help in enhancing melanoma growth and angiogenesis through ERK/p38 pathway leading to COX-2 and PGE2 production (Kale et al. 2014, Figs. 6.2 and 6.3). In adenocarcinoma specimens overexpression of OPN in infiltrating tumor-associated macrophages correlates with poor prognosis (Hsu et al. 2010). OPN secreted from macrophages reinstates the metastatic potential of hepatoma cells treated with OPN siRNA (Cheng et al. 2007). Thus, targeting macrophage derived OPN may help in regressing tumor growth.
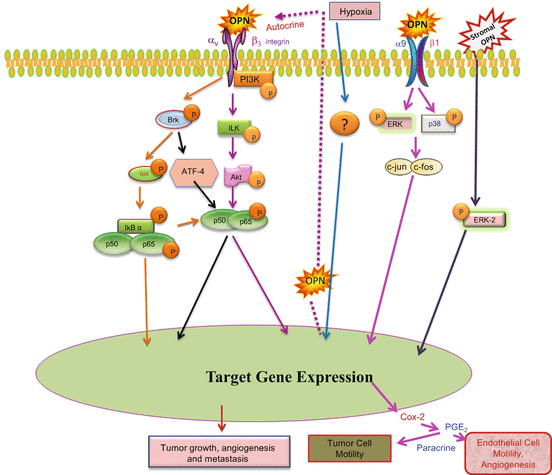

Fig. 6.3
OPN-induced signaling plays key role in tumor-stroma interaction. Under hypoxia, OPN regulates HIF-1-α and stimulates VEGF expression through ILK-/p65-dependent signaling that enhances angiogenesis and tumor progression. In macrophages, OPN induces Cox-2 and PGE2 secretion through ERK- and p38-dependent AP-1 activation via α9β1-integrin. Stromal OPN induces VEGF and ABCG2 expression from tumor cells by Brk/ATF-4 and ERK downstream signaling (Adapted from Chakraborty et al. (2008), Bandopadhyay et al. (2014))
6.4 Role of OPN and CSCs in Cancer Progression
Cancer stem cells (CSCs) are subpopulation of cancer cells that show self-renewal and differentiation properties and cancer initiating ability. CSCs are known for its chemo and radiation resistance and thus contribute to cancer recurrence after chemo and radiation therapies. CSCs also regulate various aspects of tumor progression such as angiogenesis, invasion, and metastasis. CSCs are regulated by factors derived from cancer cells as well as stromal cells. Several studies have demonstrated that stem cell properties of cancer cells are correlated with expression of OPN in tumor microenvironment (Fig. 6.2). TAMs interact with colorectal CSCs (CR-CSCs) by secreting OPN. In fact, the secretion of OPN by TAMs is regulated by CR-CSCs (Rao et al. 2013b).
In glioma, stemlike cells present in perivascular niche and they express higher levels of CD44, a well-known receptor for OPN. OPN also shows perivascular expression and it is important for the cancer stemness and radiation resistance phenotype of glioma cells. Stemlike phenotype of glioma cells is regulated by gamma secretase-regulated domain of CD44 (Pietras et al. 2014). In colon cancer, tumor-associated cells secrete OPN that increase the expression of CD44v6 in CR-CSC by Wnt-β-catenin pathway. Enhanced CD44 v6 expression promotes migration and metastasis of CR-CSCs. Hence, in colorectal cancer patients, low levels of CD44v6 expression correlate with high survival probability compared to patients with high CD44 v6 (Todaro et al. 2014). It has been demonstrated that metastasis of human breast cancer cells to the bone is related to the expression of OPN, CD44, and CXCR4 (Ling et al. 2008). These evidences suggest that OPN plays important role in regulation of cancer stem cell-mediated tumor progression.
6.5 Hypoxia: A Key Regulator of OPN in Tumor Microenvironment
Hypoxia occurs as a result of imbalance between oxygen supply and oxygen requirement to the specific tissue. Most of the solid tumors experience hypoxia when tumor grows outwards from the blood vessels resulting in oxygen deprivation. Even though hypoxia may cause death of the cancer cells, overall adaptive response against hypoxia by cancer cells helps in tumor progression, angiogenesis, and metastasis. Hypoxia also contributes either directly or indirectly to drug and radiation resistance. Hypoxia-inducible factor 1-α (HIF1-α) is a key hypoxia-responsive gene and mediates majority of the hypoxia-mediated adaptive responses. OPN is also identified as a hypoxia-responsive gene and is involved in hypoxia-mediated tumor progression (Fig. 6.2). OPN upregulates HIF1-α expression at both protein and mRNA level but it has no role in its stability. OPN-mediated HIF1-α upregulation is essential for VEGF-dependent breast tumor angiogenesis (Raja et al. 2014, Fig. 6.3). Various studies have shown that knocking down of OPN expression leads to downregulation of VEGF and HIF1-α expression. Silencing of OPN also leads to increased apoptosis, decreased invasion, as well as decreased resistance to the radiation (Yang et al. 2012a). Downregulation of OPN by BRMS1 leads to attenuation of breast cancer metastasis and increased sensitivity of the cancer cells to stress-enhanced apoptosis. Overexpression of OPN in BRMS1-transfected breast cancer cells decreases the BRMS1-mediated effects and protected the cells from hypoxia-induced apoptosis (Hedley et al. 2008).
6.6 OPN Regulates Tumor Angiogenesis
Angiogenesis is a crucial step in cancer progression as it determines the supply of oxygen and nutrients to the tumor mass. OPN promotes angiogenesis by binding to integrins through upregulating VEGF expression (Chakraborty et al. 2008, Fig. 6.3). OPN through the Brk/NF-κB/ATF4 signaling cascade can induce VEGF expression both in autocrine and paracrine manner in breast cancer cells. OPN-mediated upregulation of VEGF results in increased adhesion and migration of endothelial cells (Senger et al. 1996). Moreover, Guo et al. have demonstrated that OPN can suppress antitumor immune response through downregulation of iNOS in infiltrating immune cells (Guo et al. 2001). In the presence of inhibitor that prevents binding of RGD motif of OPN to integrins, the expression of NO increases indicating that OPN contributes towards a conductive microenvironment for tumor cells. Thrombin-cleaved OPN is biologically more active as this enable formation of a complex consisting of C-terminal of OPN, CD147 (extracellular matrix metalloproteinase inducer), and cyclophilin C leading to activation of Akt and MMPs in murine breast cancer (Mi et al. 2007). Breast cancer cells expressing thrombin-cleaved OPN exhibit decreased adhesion, apoptosis, and increased proteolytic capacity indicating that thrombin-cleaved OPN confers early tumorigenic and metastatic advantage (Beausoleil et al. 2011). Raja et al. have recently reported that under hypoxic environment OPN can induce VEGF expression through ILK-/Akt-dependent NF-κB-mediated HIF-1-α expression (Raja et al. 2014). Cowden et al. have previously shown that HIF-1-α increases expression of αvβ3 integrin thereby enhancing tumor cell invasion and migration (Cowden Dahl et al. 2005). Recent reports by Yang et al. confirmed the role of OPN, HIF-1-α, and VEGF in breast cancer angiogenesis. Silencing of OPN using siRNA and shRNA in metastatic breast cancer cells inhibits expression of HIF-1-α and VEGF eventually resulting in increased radiosensitivity of breast cancer cells (Yang et al. 2012b).
6.7 Targeting OPN in Tumor Microenvironment
In tumor microenvironment, OPN is secreted from tumor as well as stromal cells, and it is a well-pronounced regulator of the tumor instigation and progression. It regulates the expression and activation of various factors which are involved in the reciprocal interaction between tumor and stromal cells. OPN is imperative for regulating the tumor stroma to promote various hallmarks of cancer by directly engaging with the receptors on stromal cells. As a stroma-derived chemokine-like protein, OPN regulates different signaling molecules to promote cancer cell proliferation, invasion, migration, and vascularization. Hence, targeting OPN in tumor microenvironment is more effective for the treatment of cancer by employing aptamers, antibodies, small molecular inhibitors, and si/sh RNAs (Table 6.1). Blocking antibodies specific to OPN or its receptor, CD44/αvβ3, inhibits the cancer and stromal cell migration thereby obliterates the tumor progression (Mi et al. 2011; Shojaei et al. 2012; Ahmed and Kundu 2010). OPN aptamer, OPN-R3, diminishes trans-differentiation of MSCs into CAFs and thus modulates cancer metastasis (Mi et al. 2011). Small molecule inhibitors such as Andrographolide and Trichostatin-A attenuate breast cancer growth by downregulating OPN expression (Sharma et al. 2010; Kumar et al. 2012). Targeting tumor and stromal OPN by exploiting RNAi technology is more promising approach for the management of cancer (Gong et al. 2008).
Table 6.1
Therapeutic approaches for targeting tumor and stromal OPN
Agent | Nature | Target | Effect | References
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|