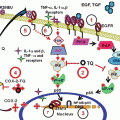
Fig. 1
(a) Formation of OSCs upon chewing/crushing of whole garlic cloves. (b) Chemical structures of commonly studied OSCs that are produced from allicin
3 Pharmacological Attributes of OSCs in Relation to Anticancer Effects
3.1 Induction of Apoptosis
The process of apoptosis is highly dysregulated in cancer cells contributing to abnormal cell growth, thus leading to increased tumor burden. OSCs have the potential of inducing apoptosis and contributing to the growth suppression of cancer cells. Sundaram and Milner (1996a, b) showed induction of apoptosis by DADS in colon cancer cells. Further studies focused on elucidation of the mechanistic details of apoptosis induction by OSCs have revealed the involvement of Bcl-2 class proteins. For example, apoptosis induction by DATS was more pronounced in prostate cancer cells PC-3 and DU-145 compared to DAS and DADS and that the induction of apoptosis was correlated with a decrease in Bcl-2 levels and reduced Bcl2:Bax interaction activating the mitochondria-mediated intrinsic pathway (Xiao et al. 2004). In the same study, it was also identified that DATS-induced hyperphosphorylation of Bcl-2 was mediated in part by c-Jun N-terminal kinases (JNK) and ERK1/2. Subsequent studies from the same group showed that DATS induces apoptosis in LNCaP prostate cancer cells by increasing Bak protein levels and decreasing Bcl-2 and Bcl-xL protein levels (Kim et al. 2007). However, ectopic expression of Bcl-2 conferred protection against DATS-induced apoptosis only in PC-3 cells and not LNCaP cells (Xiao et al. 2004; Kim et al. 2007). It was reasoned that the genotypic differences between these cells, including their p53 status and androgen responsiveness, were responsible for the observed differential effect. Similarly, in other cancer models such as lung cancer, neuroblastoma, breast cancer, and skin cancer, OSCs were shown to increase the ratio of Bax/Bcl-2, upregulating Bax protein levels and decreasing the levels of Bcl-2 and Bcl-xL proteins (Hong et al. 2000; Karmakar et al. 2007; Nakagawa et al. 2001; Li et al. 2002a, b; Wang et al. 2010, 2012a, b).
It is interesting to point out that OSCs have little or no effect on normal cells, although the mechanism underlying their selectivity for cancer cells is not completely understood. For example, the PrEC normal prostate epithelial cells were more resistant to DATS-induced apoptosis than prostate cancer cells (Kim et al. 2007). Similarly, DAS or DADS administration induced apoptosis in SH-SY5Y neuroblastoma cells and did not impact the viability of primary neurons (Karmakar et al. 2007). In a different study, ajoene caused apoptotic cell death in human leukemia cells but had no effect on normal peripheral mononuclear blood cells (Dirsch et al. 1998).
3.2 Modulation of Carcinogen Metabolism
Multiple studies indicate that chemopreventive effect of OSCs is at least in part due to their ability to inhibit the activation of carcinogens and/or increase detoxification of the activated metabolites. N-nitrosodimethylamine (NDMA) is a by-product of industrial processes, while 4-(methylnitrosamino)1-(3-pyridyl)-1-butanone (NKK) is a carcinogen found in tobacco smoke. Their toxicity and carcinogenicity is dependent upon activation by the Phase 1 drug-metabolizing enzyme, CYP 2E1. Early studies utilizing in vitro cell culture and in vivo animal models revealed that OSC treatment prevented cytotoxicity and tumor formation induced by NDMA and NKK (Hong et al. 1992). Furthermore, it was suggested that OSCs inhibit P450 2E1 by both competitive inhibition and suicide inactivation (Brady et al. 1991). OSCs may also protect against acetaminophen toxicity in mice via inhibition of CYP2E1 (Wang et al. 1996). More recent evidence confirmed that oral OSC treatment at 100–400 mg/kg depressed CYP2E1 activity in male Sprague–Dawley rats in a dose-dependent manner. This correlated with statistically significant reductions in serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activity following treatment with the hepatotoxicant thioacetamide (Kim et al. 2014), indicating a reduction in liver damage.
Experimental evidence suggests that OSCs may also enhance the detoxification of carcinogens via induction of Phase 2 drug-metabolizing enzymes. One recent study confirmed that oil-soluble garlic compounds activate metabolizing enzymes that detoxify carcinogens. This resulted in a reduction of the development of mammary cancer in animals and suppression of growth of human breast cancer cells in culture (Tsubura et al. 2011). Earlier studies demonstrated the ability of OSCs to prevent benzo(a)pyrene (BP)-induced for stomach tumorigenesis in mice. The authors attributed this to increased expression of NAD(P)H:quinone oxidoreductase (NQO), an enzyme implicated in the detoxification of activated quinone metabolites of BP (Singh et al. 1998). Munday and Munday (2001) documented increases in the activity of the Phase II enzymes NQO and glutathione-S-transferase (GST) in rat tissues following oral doses of both DADS and DATS. OSCs enhanced glutathione content of intestinal mucosa and liver in irradiated Swiss albino mice (Chittezhath and Kuttan 2006). Oil-soluble OSCs induced GST phosphorylation via activation of c-Jun NH2-terminal kinase (JNK) in neuroblastoma cells (Filomeni et al. 2003). Studies have confirmed activation of GST by JNK in normal rat liver cells (Tsai et al. 2011).
In summary, OSCs act as a double-edged sword in chemoprevention by inhibiting carcinogen activation by Phase 1 enzymes while simultaneously enhancing detoxification of activated carcinogenic intermediates via induction of Phase 2 enzymes (Herman-Antosiewicz et al. 2007).
3.3 Inhibition of Cell Cycle Progression
Numerous studies have demonstrated the antiproliferative effects of DATS in a variety of cancer cell types, including liver, gastric, colon, prostate, lung, bladder, and skin cancer cells. The DATS appear to induce cell cycle arrest in the G2/M phase; however, the mechanism by which this occurs may be cell-type specific (for review see Antony and Singh 2011; Yi and Su 2013). In colon cancer cells, DADS-induced G2/M phase arrest was associated with hyperphosphorylation and decreased expression of cell division cycle 25C phosphatase (Cdc25C), inhibition of cdc2 kinase activation, and decreased formation of cdc2/cyclin B1 complex formation (Knowles and Milner 2000). Similarly, Xiao et al. (2005) demonstrated destruction and hyperphosphorylation of Cdc25C and inhibition of cdc2/cyclin B1 kinase activity in prostate cancer cells upon treatment with DATS. This effect was dependent upon generation of reactive oxygen species (ROS). Wang et al. (2010) demonstrated increased ROS generation in DATS-treated skin cancer cells. ROS production associated with G2/M arrest was also seen in neuroblastoma (Filomeni et al. 2003), colon (Song et al. 2009), and lung (Wu et al. 2009) cancer cells.
A role for checkpoint 1 kinase (Chk1) in OSC-mediated cell cycle arrest was also proposed. Chk1 is normally activated in response to DNA damage and results in cell cycle arrest prior to DNA repair or apoptosis. It has been found that SATS-induced mitotic arrest is dependent upon activation of Chk1 in prostate cancer cells and gastric cancer cells (Herman-Antosiewicz and Singh 2005; Ling et al. 2010).
Cell cycle arrest in liver tumor cells was associated with decreased cyclin-dependent kinase 7 protein levels and increased cyclin B1 protein levels (Wu et al. 2004). OSC-induced mitotic arrest in human leukemic cells was attributed to activation and nuclear translocation of nuclear factor-κB (NF-κB) followed by NF-κB binding to the promoter region of cyclin-dependent kinase inhibitor 1 (p21) (Dasgupta and Bandyopadhyay 2013). OSC induced upregulation of p21 was also demonstrated in a study utilizing colon cancer cells (Liao et al. 2009). Multiple studies have associated mitogen-activated protein kinase (MAPK) activation with G2/M arrest. DADS have been shown to trigger cell cycle arrest in both colon cancer (Knowles and Milner 2003) and lung carcinoma cells (Hui et al. 2008). In both studies, this was associated with increased activity of extracellular signal-regulated kinase (ERK). In contrast, DADS-mediated arrest in gastric tumor cells was associated with upregulation of p38 MAPK (Yuan et al. 2004). Finally, several studies have identified OSC binding sites on components of tumor cell cytoskeleton and correlated morphological changes to the cytoskeleton with G2/M arrest (Hosono et al. 2005; Xiao et al. 2005; Aquilano et al. 2010).
Cell cycle arrest, if sustained, provides a powerful preventative mechanism to the growth of tumors both in vitro and in vivo. In some of the studies cited above, cell cycle arrest was transient, but was then followed by apoptosis (Xiao et al. 2005; Song et al. 2009; Aquilano et al. 2010; Dasgupta and Bandyopadhyay 2013). Regardless of the mechanism by which it occurs, cell cycle arrest appears to be a common pharmacological effect of OSCs in cancer cells.
3.4 Induction of Reactive Oxygen Species
Accumulating evidence suggests a role for reactive oxygen species (ROS) in cancer cell apoptosis by OSCs. DADS-induced apoptosis in SH-SY5Y neuroblastoma cells was associated with ROS generation (Karmakar et al. 2007). Likewise, ajoene-induced apoptosis in human promyelocleukemic cells was linked to ROS and activation of NF-κB (Dirsch et al. 1998). Studies involving prostate cancer cells (Kim et al. 2007), basal cell carcinoma (Wang et al. 2012a, b), MCF-7 breast cancer cells (Na et al. 2012), and leukemia cells (Choi and Park 2012) also reported that DATS-induced apoptosis was mediated through ROS generation.
The precise mechanism by which DATS causes ROS generation is not fully understood, but some advances in this context are worthy of discussion. For example, apoptosis induction and cell cycle arrest by DADS and DATS were shown to be mediated through increased intracellular calcium (Park et al. 2002; Wang et al. 2010). Cell cycle arrest and apoptosis induction by DATS in prostate cancer cells was shown to be mediated by ROS, generated through increase in labile iron due to proteasomal degradation of ferritin (Antosiewicz et al. 2006). Wang et al. (2010) showed that DATS-dependent calcium increase in basal cell carcinoma (BCC) cells was accompanied by intracellular ROS generation. The ROS generation by OSCs was also linked with the generation of hydrogen sulfide (H2S) where it was shown that OSCs can be converted to H2S in human red blood cells (Benavides et al. 2007). More recently, it was shown that DATS-induced H2S is associated with the generation of ROS and activation of mitochondria-mediated apoptosis pathway in human breast cancer MCF-7 cells (Na et al. 2012). Based on these studies, it is evident that OSC-dependent ROS generation is crucial for their anticancer effects.
3.5 Inhibition of Angiogenesis and Cell Invasion
Inhibition of angiogenesis, which is required for tumor growth, is another major effect of some OSCs. Mousa and colleagues demonstrated the anti-angiogenic potential of alliin as it inhibited the tube formation, dependent on fibroblast growth factor-2 and vascular endothelial growth factor (VEGF) both in human endothelial cells and in a chick chorioallantoic membrane assay (Mousa and Mousa 2005). In the same study, the authors also showed that the anti-angiogenic potential of alliin, which was in part mediated by increase in cellular nitric oxide and p53 protein expression, increased in the presence of vitamin C and vitamin E. The DATS-associated anti-angiogenic properties were examined in human umbilical vein endothelial cells (HUVEC). DATS was shown to inhibit formation of capillary-like tube structure and migration by HUVECs. Mechanistic details revealed suppression of VEGF secretion, downregulation of VEGF receptor-2 protein level, and inactivation of Akt upon treatment with DATS (Xiao et al. 2006a, b).
The effect of OSCs on migration and invasion of cancer cells has also been studied. Ajoene administration inhibited B16/BL6 melanoma cell adhesion to LEC1 cells in vitro and also significantly inhibited lung metastasis in C57BL/6 mice injected with B16/Bl6 melanoma cells (Taylor et al. 2006). Similarly, DADS treatment resulted in the inhibition of HUVEC cell proliferation and activity of matrix metalloprotease-2 (MMP-2) and MMP-9 (Meyer et al. 2004). The DATS administration in a transgenic mouse model of prostate cancer (Transgenic Adenocarcinoma of Mouse Prostate; TRAMP) not only prevented the development of poorly differentiated prostate cancer but also inhibited pulmonary metastasis multiplicity (Singh et al. 2008). In a study using osteosarcoma cells, DATS was shown to exhibit antitumor activity by targeting Notch1 signaling and inhibiting cell invasion and angiogenesis partly through downregulation of VEGF, MMP-2, and MMP-9 (Li et al. 2013). DAS, DADS, and DATS were shown to inhibit migration, invasion, and angiogenesis. For example, exposure of human colon cancer Colo 205 cells to all three OSCs resulted in inhibition of PI3K, Ras, MEKK3, MKK7, ERK1/2, JNK1/2, and p38 which correlated with the inhibition of MMP-2, -7, and -9, essential for cell migration and invasion (Lai et al. 2013). DATS was also shown to inhibit mRNA and protein levels of VEGF and MMP-2, -7, and -9 in human colon cancer HT29 cells (Lai et al. 2015). In addition to reducing MMP levels, inhibitory effects of DADS in Colo 205 cells and LNCaP cells were also found to be associated with reduced levels of proteins associated with tight junctions functionality [Lai et al. (2013), Shin et al. (2010), for review see Yi and Su (2013)].
3.6 Immunomodulation
Immunomodulatory effects of garlic and its OSCs have been reported (for a review, see Schafer and Kaschula 2014). Studies have identified that garlic and its constituents can effectively strengthen the host immune system within the tumor against the immunosuppressive activity of an emerging tumor (Schafer and Kaschula 2014). Aged garlic extract along with an anticancer agent suppressed tumor growth of sarcoma-180 and Lewis Lung carcinoma LL/2 cells injected in mice by inducing cellular immune response through the activation of NK cells and cytotoxic T cells (Kyo et al. 1998). In addition, aged garlic extract was also found to stimulate lymphocyte proliferation, macrophages phagocytosis, and lymphocyte infiltration into tumors and enhanced NK cell number and activity (Schafer and Kaschula 2014). A recent study showed that intra-tumor inoculation of a protein fraction of fresh garlic bulbs was more efficient than garlic extract in infiltrating the tumor with CD8+ T cells (Ebrahimi et al. 2013). DADS downregulated the levels of CCL-2 (an important chemokine which favors tumor cell migration and expansion) induced by TNF-α in MDA-MB 231 breast cancer cells (Bauer et al. 2014). It was reasoned that CCL-2 release by breast cancer cells may be regulated by pro-inflammatory cytokines through NF-κB or ERK.
Even though studies have identified conflicting evidence (for a review, see Schafer and Kaschula 2014) with the immunomodulation properties of OSCs, it is noteworthy to mention that immunomodulatory effects of OSCs may contribute to their overall anticancer activity.
3.7 Modulation of Histone Acetylation
Balance between histone acetylation and deacetylation is crucial for regulation of gene expression essential for normal cellular processes. However, this balance is often lost in cancer cells, favoring their uncontrolled growth and progression. Discovery of agents that can either block the activity of histone deacetylases (HDACs) or promote the histone acetylation activity is crucial in combating cancer development. There are examples of clinical success of this approach (vorinostat). Studies have shown that garlic and its compounds have the potential of both increasing histone acetylation and inhibiting HDAC activity (for reviews, see Druense-Pecollo and Latino-Martel 2011; Yi and Su 2013). For example, DADS caused an increase in the acetylation of histones H3 and H4 in DS19 and K562 human leukemic cells. Acetylation was also induced in rat hepatoma and human breast cancer cells by DADS and its metabolite, allylmercaptan. Moreover, in the same study it was shown that allylmercaptan was more potent in inhibiting HDAC compared to DADS (Lea et al. 1999). The induction of histone acetylation by S-allylmercaptocysteine (SAMC), allicin, and DADS in various cancer models was also shown (Lea and Randolph 2001; Lea et al. 2001). DADS was also shown to promote cellular accumulation in G2/M phase by decreasing HDAC activity and increasing histone H3 and H4 acetylation, thus causing an increase in p21 mRNA and protein levels in colon cancer CaCo-2 and HT29 cells (Druesne et al. 2004). A recent study showed that DATS-induced acetylation of histones H3 and H4 and inhibition of HDAC activity contributed to the inhibition of glioblastoma xenograft growth (Wallace et al. 2013).
4 Preclinical In Vivo Evidence for Chemopreventive Effects of OSCs
A wealth of literature exists characterizing effects of Allium-derived OSCs on prevention of cancer initiation and promotion. The primary compounds investigated include the oil-soluble DATS, DADS, DAS, and ajoene, and the major water-soluble component SAC. With the exception of ajoene, these compounds have received roughly equal attention in the literature.
4.1 DATS
Although other OSCs have received significant attention for their chemopreventive ability against carcinogens, the majority of preclinical studies with DATS used rodents that either received a cancerous cell implant or were genetically susceptible to cancer. The major cancers investigated with DATS include lung, colon, liver, prostate, skin, and breast, with a predominance of prostate cancer studies. These studies were initiated in 2005 (Table 1).
Table 1
Preclinical studies on DATS
Lead author | Year | Animal | Cancer | Carcinogen | Treatment | Results |
|---|---|---|---|---|---|---|
Hosono | 2005 | Mice (female nude) | Colon: HCT-15 xenograft s.c. | No | 6 mg/kg via tail vein q3d from day 7 to 25 | Prevented colon tumors (P value not reported but tumor volume was on average < 1/3 of control after 25 days) |
Xiao | 2006 | Mice (male athymic) | Prostate:PC-3 cell s.c. xenograft | No | Oral gavage 6 μmol, 3×/week | Tumor growth was slowed without causing weight loss (3-fold smaller in 20 days). DATS caused more apoptotic bodies and more Bax and Bak expression (pro-apoptotic proteins), but did not inhibit angiogenesis |
Zhang | 2007 | Mice (BALB/c nude) orthotopic transplantation | Liver: HCC HepG2 xenografts into liver | No | Liver-targeted intravenous polybutylcyanoacrylate nanoparticles of DATS (1.5 mg/kg qod, 2 weeks) | DATS-nano. distributed differently than DATS alone (highly liver targeted, while DATS alone showed renal predominant targeting. Both targeted spleen similarly and substantially). DATS-nano retarded growth of hepatoma compared to DATS, nano., or saline, with no weight loss. PCNA and Bcl-2 proteins were downregulated in DATS-nano |
Singh | 2008 | Mice (TRAMP) | Prostate and Lung | No | Oral gavage 1 and 2 mg/day, 3×/week, 13 weeks | Inhibited progression to poorly differentiated prostate CA and pulmonary metastasis multiplicity (not incidence of metastasis) w/out side effects. Dorsolateral prostate showed decreased cell proliferation from DATS and induction of cyclinB1 and securin protein. DATS did not increase apoptosis or affect angiogenesis or natural killer or dendritic cell function |
Shankar | 2008 | Mice (BALB/c nude) | Prostate: orthotopic PC-3 cell implant into prostate | No | Oral DATS (daily 5days/week 40 mg/kg) | Inhibited growth of prostate CA without causing weight loss. Co-treatment w/DATS and TRAIL was more effective at growth inhibition, DR4 and DR5 protein induction, caspase-8 activation, and apoptosis induction than either agent alone. DATS inhibited angiogenesis and metastasis-related protein expression and Akt and NF-kB activation. This effect was stronger when combined with TRAIL |
Stan | 2009 | Mice (TRAMP) | Prostate | No | Oral gavage of DATS (2 mg/day, 3×/week, 13 weeks) | Suppressed androgen receptor protein expression in poorly differentiated prostate cancer |
Chandra-Kuntal | 2010 | Mice (TRAMP) | Prostate | No | The 2 mg-treated mice from (Singh et al. 2008) | The reduction in poorly differentiated prostate tissue correlated with a decrease in the oncogenic protein pSTAT3. When STAT3 was made constitutively active in cells, the response to DATS was not attenuated. Thus, inhibition of STAT3 activation alone is not required for DATS’s pro-apoptotic effect |
Shrotriya | 2010 | Mice | Skin | DMBA and TPA | Pretreatment of skin (25 μmol topical) 30 min before TPA | DATS more effective than other allyl sulfides (DATS > DADS > DAS) in suppressing TPA-induced COX-2 expression. DATS reduced DNA binding of activator protein 1 (AP-1), a transcription factor for COX-2. DATS also reduced JNK and Akt activation. DATS significantly reduced incidence and multiplicity of papillomas |
Wu | 2011 | Mice (female BALB/c) | Colon: i.p. CT-26 cell allograft | No | DATS (10 or 50 mg/kg) i.p. q4d 4 weeks prior to cell inoculation | 50 mg/kg reduced tumor volume and weight significantly |
Kim | 2011 | Mice (TRAMP) | Prostate | No | 2 mg/day/mouse, 3×/week for 13 weeks beginning at 8 weeks of age | Dorsolateral prostates from treated mice showed significant downregulation of XIAP and induction of Survivin protein vs. control mice. Ectopic expression of XIAP partially reversed the DATS-induced apoptosis |
Li | 2012 | Mice (female BALB/c nude) | Lung: A549 cell s.c. xenografts | No | Oral gavage, 6 μM in 100 μl PBS qod (0.6 nmol total mass) for 30 days | Slowed growth of xenografts with no apparent side effects (no weight loss) |
Na | 2012 | Mice (BALB/c female) | Breast: thoracic implant w/human MCF-7 Breast CA cells | 17-β-estradiol implanted device | Oral (5 μmol/kg, 2×/week for 1 month) | Inhibited growth of xenografts. In cells, DATS induced apoptosis that was dependent on JNK. DATS-induced cell apoptosis and JNK activation was reduced by N-acetyl-l-cysteine (NAC). DATS increased DNA binding of AP-1, which was blocked by NAC or JNK inhibitor |
Female BALB/c nude mice with human lung adenocarcinoma cell (A549) xenografts showed significantly retarded tumor growth with no apparent side effects when given a DATS oral gavage of 0.6 nmol every other day for 30 days (Li et al. 2012). Singh et al. (2008) studied the ability of oral DATS (1 and 2 mg/day, thrice weekly for 13 weeks) to inhibit lung metastasis from prostate cancer in TRAMP mice. DATS administration did not reduce the incidence of metastasis, but multiplicity was lower in the treatment group compared with control.
Female nude mice subcutaneously implanted with human colon cancer cells (HCT-15) exhibited a marked reduction in tumor volume relative to control after 25 days of DATS treatment at 6 mg/kg IV every 3 days initiated 7 days after xenograft implantation (Hosono et al. 2005). Statistical significance was not reported, but average tumor volume was less in the treatment group versus control mice. Wu et al. (2011) found that female BALB/c mice with mouse colon carcinoma cell (CT-26) allografts had significantly reduced tumor volumes and weights when administered DATS at 50 mg/kg by oral gavage every 4 days starting 4 weeks prior to cell inoculation.
In a unique study by Zhang et al. (2007), polybutylcyanoacrylate nanoparticles containing DATS were tested for 2 weeks of treatment against orthotopically transplanted HepG2 liver cancer cells. The subcutaneously grown tumors were subsequently implanted under the envelope of the liver in BALB/c nude mice. IV DATS or DATS-filled nanoparticles were injected every other day for 14 days at 1.5 mg/kg. The DATS nanoparticles were markedly more liver targeted than DATS alone, which showed predominantly renal localization. Both formulations showed significant spleen distribution. Moreover, DATS nanoparticles retarded growth of liver tumors more than DATS with no weight loss. To determine a molecular basis for the result, PCNA and Bcl-2 proteins were assessed. Both were downregulated in the tumors from DATS nanoparticle-treated mice compared with control tumors.
In preclinical prostate cancer studies on DATS, investigators mostly employed the TRAMP model or grown xenografts with PC-3 cell implantation. Studies with TRAMP mice focused on changes in protein expression of cell growth and apoptosis related proteins, while the follow-up ones directly assessed effectiveness against tumor growth. Kim et al. (2011) found that DATS given at 2 mg/day thrice weekly for 13 weeks caused significant downregulation of XIAP while inducing survivin protein in TRAMP mice. Ectopic expression of XIAP partially reversed DATS-induced apoptosis in prostate cancer cells, strengthening the contention that DATS-induced XIAP downregulation is important for induction of apoptosis. Stan and Singh (2009) demonstrated that the same dose and schedule of DATS suppressed androgen receptor (AR) protein expression in poorly differentiated prostate cancer in TRAMP mice. Therefore, there is evidence indicating that DATS can both trigger apoptosis and attenuate prostate cancer-promoting receptor expression.
Oral gavage of DATS in TRAMP mice at either 1 or 2 mg/day, three times a week for 13 weeks, significantly inhibited progression to poorly differentiated prostate cancer, and showed a trend toward a reduction in prostate weight, albeit not significant (Singh et al. 2008). Dorsolateral prostate showed reduced cell proliferation from DATS treatment and induction of cyclinB1 and securin protein. DATS did not increase apoptosis or affect angiogenesis. In Chandra-Kuntal and Singh (2010), mice from the same study were further assessed. The reduction in poorly differentiated prostate cancer cells from the 2 mg DATS treatment group in Singh et al. (2008) correlated with a decrease in phosphorylated STAT3, an oncogenic protein. However, forced expression of STAT3 in prostate cancer cells did not attenuate the response to DATS, indicating that inhibition of STAT3 activation is not required for the preventive effect of DATS.
Xiao et al. 2006a, b found that oral DATS at 6 μmol thrice weekly significantly slowed tumor growth in male nude mice subcutaneously implanted with PC-3 cells. Tumors were 2/3 smaller in treated mice in 20 days vs. untreated mice. DATS caused more apoptotic bodies and increased expression of Bax and Bak (pro-apoptotic proteins). However, DATS did not inhibit angiogenesis, and was apparently not toxic, as demonstrated by lack of weight loss. Shankar et al. (2008) approached PC-3 cell implantation differently by implanting directly into the prostate rather than studying the cells’ subcutaneous growth. They found that oral DATS given every day 5 days a week at 40 mg/kg inhibited growth of the prostate cancer implant vs. control. When DATS was combined with TRAIL (tumor necrosis factor-related apoptosis-inducing ligand), the combination was even more effective at growth inhibition than DATS. TRAIL-R1/DR4 and TRAIL-R2/DR5 protein induction, caspase-8 activation, and apoptosis induction were also greater with the combination than either agent alone. DATS also inhibited angiogenesis and metastasis-related protein expression and Akt and NF-κB activation, but again did so more strongly when combined with TRAIL (Shankar et al. 2008).
The two-stage murine model of skin cancer with DMBA (dimethylbenz[a]anthracene) as the initiator and TPA (tetradecanoylphorbol-13-acetate) as the promoter was employed to test the cancer protective effect of DATS (Shrotriya et al. 2010). Topical DATS at 25 μmol 30 min before TPA application was effective in suppressing TPA-induced COX-2 expression. COX-2 suppression appeared to have been caused by a DATS-triggered reduction in DNA binding of activator protein 1 (AP-1), a transcription factor for COX-2. DATS also reduced JNK and Akt activation, and moreover, reduced the incidence and multiplicity of skin papillomas. COX-2 has been linked with skin cancer through its inflammatory role in the body, thus providing a mechanistic explanation for the reduced papilloma formation.
Na et al. (2012) employed BALB/c mice implanted with human breast cancer cells (MCF-7) directly into the thoracic area and promoted with estradiol-releasing implanted devices. Oral DATS at 5 μmol/kg twice weekly for 1 month inhibited xenograft growth.
Cell studies indicated that DATS induced apoptosis that was dependent on JNK. DATS increased DNA binding of AP-1. This may appear to be in contrast to the skin cancer study by Shrotriya et al. (2010) with respect to AP-1 effects; however, in Shrotriya et al. (2010), DATS’s effects on AP-1 DNA binding were assessed in the context of TPA-promoted skin cancer, whereas in Na et al. (2012) DATS’s effects on AP-1 DNA binding were assessed in the presence of estradiol-promoted breast cancer. Thus, DATS effects on AP-1 are likely context/environment dependent.
4.2 DADS
DADS have been assessed in treatment or prevention of cancers of the skin, mammary tissue, liver, colon, kidney, and stomach, in addition to leukemia. Earlier studies generally employed cancer initiators and promoters (two-stage model), whereas later studies relied heavily on xenografts of various cancer cell types (Table 2).
Table 2
Preclinical studies on DADS
Lead author | Year | Animal | Cancer | Carcinogen | Treatment | Results |
|---|---|---|---|---|---|---|
Dwivedi | 1992 | SENCAR (two-stage model-sensitive) Mice | Skin | DMBA and TPA | Topical DADS and DAS (1 mg/100 μl) administered 30 min before DMBA and 30 min before TPA | Inhibited skin papilloma formation from the 9th week of promotion and increased survival |
Ip | 1992 | Rats | Mammary | DMBA | Gavage 1.8 mmol/kg at 96, 48, and 24 h before 10 mg intragastric DMBA, single dose | Protected in the initiation phase (61 % reduction in tumor incidence and 56 % reduction in total tumor number), somewhat more so than DAS |
Takahashi | 1992 | Rats (male F344) | Liver, colon, kidney: two-step cancer models | N-diethylnitrosamine i.p. (200 mg/kg) and other carcinogens sequentially | DADS (50 mg/kg) or DAS (200 mg/kg) gavage, 3×/week for 6 weeks after NDN, or for 24 weeks after admin. of other carcinogens | DADS inhibited colon and renal CA. DAS increased levels of a liver cancer-associated protein, but an increased risk for liver cancer was not confirmed |
Sundaram | 1996 | Mice (female) | Colon: human colon tumor cell line s.c. xenograft, HCT-15 | No | 1 mg 3×/week by gavage or i.p. | I.p. Reduced tumor volume by 69 % without apparent toxicity. Gavage was also effective, but less than i.p.. DADS was as efficacious as 5-FU. DADS and 5-FU combined was no more effective than either alone. DADS also reduced 5-FU toxicity by unknown mechanism |
Singh | 1996 | Mice (BALB/c nude) | Subcutaneous implant of H-ras oncogene-transformed NIH 3T3 (fibroblast) cell xenograft | No | Oral administration (33 μmol thrice weekly) | Delayed and inhibited tumor growth. Inhibition of tumor growth correlated with inhibition of p21H-ras membrane association. DADS also inhibited hepatic and tumoral HMG-CoA reductase. Suggested that inhibition of prenylation may be the therapeutic mechanism
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|