Key points
- 1.
Minimally invasive surgery (MIS) is practiced by more than 90% of gynecologic oncologists.
- 2.
Knowledge of anatomy, the disease process, and surgical technique is key during these complicated surgical procedures.
- 3.
Several studies have shown that 10 to 20 cases are needed to gain proficiency with a certain procedure.
- 4.
MIS reduces blood loss, transfusions, length of hospital stay, and wound complications without compromising adequacy of the procedure or staging even in (extremely) morbidly obese patients.
- 5.
Survival outcomes for endometrial cancer is similar after MIS and laparotomy, while survival outcomes with MIS have been shown to be worse for cervical cancer.
- 6.
Several safe methods exist to extract an enlarged uterus after MIS. Morcellation is not recommended when there is suspicion or proven preinvasive or invasive disease.
- 7.
Laparoscopy can be used in ovarian cancer to assess the extent of disease and chance of complete debulking surgery. MIS in advanced or recurrent ovarian cancer is increasingly utilized for tumor resection, and prospective trials to confirm safety and feasibility are ongoing.
Laparoscopic surgery in gynecologic oncology
Recent advances in the techniques of minimally invasive surgery (MIS) have greatly expanded its role in the management of gynecologic malignancies. Before the 1990s, MIS was mostly limited to laparoscopy (LS) for diagnosis of pelvic disease and for tubal sterilization procedures. The great majority of gynecologic oncology procedures for definitive surgical management were performed via large midline abdominal incisions to accomplish appropriate extirpation of the malignancy and surgical staging. Laparotomy causes significant trauma to the patient with many potential associated morbidities, which are increased in incidence and severity in patients with comorbid conditions. The applications for MIS in patients diagnosed with gynecologic malignancies have gradually expanded over the past 20 years with improvements in video-laparoscopic instrumentation and surgical training. Since 2005, advances in robotic surgery have led to the increased use of MIS for comprehensive surgical management of patients with gynecologic cancers. Most gynecologic oncologists now offer MIS as an option for surgical management of patients diagnosed with endometrial and ovarian neoplasia and for select cervical cancer cases.
Advanced laparoscopic procedures have been an option for a subpopulation of gynecologic oncology patients since the 1990s. The goal of MIS is to decrease patient discomfort, hospital stay, and short- and long-term morbidity while at the same time providing an overall improvement in quality of life (QoL) and allowing for earlier implementation of other adjuvant therapies if necessary. Significant advances in instrumentation and laparoscopic technology, including high-definition cameras and fluorescence imaging, have increased the role of MIS in gynecologic oncology. Advanced robotic technologies have expanded the ability of surgeons to offer minimally invasive procedures in settings that may not have otherwise been feasible, including super morbid obesity and extensive prior surgical history. Trainees completing gynecologic oncology fellowship have significant experience with MIS, increasing the role of robotic laparoscopic surgery in gynecologic cancer.
As illustrated in other chapters in this textbook, the evolution of surgery in patients with gynecologic cancers has a long history. The advancements include the incorporation of modifications to radical surgical procedures that reduce the toxicities of treatment with the goal of obtaining the optimal oncologic outcome while improving overall patient QoL. The goals of cancer control and patient safety must not be compromised by a given surgical approach. In other words, MIS serves as another tool to achieve these goals rather than a separate end in itself. The historical advantages of laparotomy compared to LS include maximal surgical exposure, three-dimensional (3D) vision, direct tissue palpation and manipulation, and ease of suturing, and other instrument use. Improvements in MIS technology, such as the robotic platform and 3D LS, have allowed MIS to get a step closer to the surgical experience with laparotomy. In addition, MIS has improvement in patient-centered outcomes, including reduced blood loss, decreased postoperative pain, less risk of infection, and shorter postoperative hospital stays. Existing clinical data has shown that MIS is feasible for the most common gynecologic cancers. Ongoing studies will provide insight into cancer-specific outcomes such as disease-free and overall survival to ensure the best surgical approach is chosen. Regardless of the surgical approach, it is still imperative that the surgeon adheres to the primary surgical principles of optimal exposure, meticulous tissue dissection, expert knowledge of anatomy, and an understanding of the natural history of the diseases being treated to overcome any potential compromise.
Laparoscopic surgical staging of gynecologic malignancies
The cornerstone of appropriate surgical staging is an accurate pelvic and paraaortic lymph node dissection. Incorporation of MIS into the surgical management of patients with gynecologic cancer was not feasible until a technique for adequate laparoscopic lymph node dissection was made possible. Pioneering descriptions of laparoscopic procedures for pelvic lymph node (PLN) dissection came from Europe in the late 1980s by Dargent and Querleu and coworkers. Although exciting in terms of the prospects of MIS, there were concerns regarding the adequacy of lymph node dissection, operative risks, and ability to access the paraaortic nodes. Since that time, laparoscopic skills have evolved, and this approach has been demonstrated to be feasible with many descriptions of the surgical technique and outcomes of the laparoscopic pelvic and paraaortic lymph node dissections now reported. A growing body of data exists demonstrating safety and feasibility of laparoscopic lymphadenectomy in the treatment of gynecologic cancers. With the advent of sentinel lymph node (SLN) dissection for gynecologic cancers, preliminary evidence demonstrates sentinel lymphadenectomy has less risk of postoperative complications compared to standard laparoscopic or robotic lymphadenectomy. Prospective trials will help elucidate when sentinel lymphadenectomy can be used to accurately assess metastatic disease in gynecologic cancers.
Robotic surgery in gynecologic malignancies
Robotic surgery overcomes many of the technologic limitations of LS. The robotic platform provides the surgeon with superior high-definition 3D vision, magnification, wristed instruments, and motion scaling. The surgeon has much more ability to directly control the operative field compared with traditional LS, thus eliminating many important disadvantages of LS. The operator of the robotic platform not only has improved vision but also controls the direction and distance of the camera from the operative field without relying on the assistant. In addition, the surgeon has three other port sites to use for a dissector, cutting instrument, and another retracting instrument. Loss of haptic feedback is a potential disadvantage; however, the experienced surgeon is able to overcome loss of this sense with heightened visual feedback and meticulous surgical technique. In addition, there are no significant ergonomic disadvantages, and a reduced risk of injury to the surgeon and much less risk of fatigue has been demonstrated in some studies. These significant technological improvements have allowed the gynecologic surgeon to perform much more complicated surgeries via MIS on a heterogeneous group of patients. More than fifteen years after the Food and Drug Administration (FDA)’s approval of robotic surgery for gynecologic surgery (2005), many publications have confirmed the safety and feasibility of robotic surgery compared with traditional LS and laparotomy. The initial series focused mostly on staging procedures for endometrial cancer; however, the experience has evolved to more advanced procedures such as radical hysterectomy, ovarian cancer debulking surgery, and pelvic exenteration. It is now the dominant MIS surgical approach in gynecologic oncology.
Length of stay
Initial studies of MIS for gynecologic cancer reported decreased length of stay from 4 days to 2 days compared to laparotomy. With the increasing use of MIS, surgeon and patient comfort, and improvements in perioperative management, length of stay has been further reduced. Especially with the implementation of the Enhanced Recovery After Surgery (ERAS) pathways, which incorporates preoperative nutrition and hydration, peri-operative oral pain medications (often a combination of acetaminophen, nonsteroidal antiinflammatory drugs [NSAIDs], a narcotic, and gabapentin), along with judicious intraoperative fluid management, early postoperative ambulation, and nutrition. This has resulted in an even shorter length of stay of one day or same day discharge, without increasing readmissions or complications, even in patients with multiple comorbidities.
Minimally invasive surgery learning curve
Advances in the techniques of MIS have greatly expanded its role in the management of gynecologic malignancies. Based on several reports, it likely requires approximately 20 to 25 laparoscopic endometrial cancer cases to gain proficiency in this procedure, but individual surgeon experience and outcomes are quite heterogeneous. In the largest single-institution report on transperitoneal laparoscopic pelvic and paraaortic lymph node dissection, Schneider and colleagues estimated 20 operations were required to gain the needed experience for laparoscopic pelvic lymphadenectomy and up to 100 for paraaortic lymphadenectomy.
The robotic platform is being more rapidly adapted by gynecologic oncologists to perform extrafascial and radical hysterectomies with pelvic and paraaortic lymph node dissections and is similarly associated with a learning curve. Seamon and colleagues determined that proficiency for hysterectomy with pelvic and paraaortic lymph node dissection in women with endometrial cancer is achieved at 20 cases, and further efficiency continues to improve over time.
In a prospective, randomized trial, Coleman and Muller reported significant improvement in laparoscopic proficiency in residents exposed to a laboratory-based skills curriculum. Training specifically geared toward laparoscopic surgery using models, cadavers, and animal laboratories is important in gaining proficiency in advanced laparoscopic surgery. The learning curve for robotics does appear to be distinctly different from that for LS, as investigators have demonstrated that surgical drills and suturing are performed with enhanced precision and dexterity when comparing robotic technologies with LS in a training laboratory. In a 2002 survey of LS training among Society of Gynecologic Oncology (SGO) members, 85% reported receiving no or limited laparoscopic training during their fellowship. A follow-up survey in 2009 among members of the SGO demonstrated that 91% performed laparoscopic surgery in their practices even though 76% reported that they had limited or no exposure to laparoscopic training during fellowship. Ninety-seven percent of gynecologic oncologists now perform robotic surgery. The introduction of simulators, formal resident and fellow training, and the dual-console da Vinci system (Intuitive Surgical) in 2009 allow for a safe training environment and similar patient outcomes compared with traditional laparoscopic surgery. The transition from LS to robotic surgery for the MIS management of gynecologic cancers will likely further diminish experience in laparoscopic surgery in this population. There may be an advantage to the robotic platform for novice minimally invasive surgeons, given the ability for higher magnification and 3D vision. However, any novel technology does not obviate the need for sound surgical principles and technique, knowledge of anatomy, and understanding of the natural history of the diseases being treated.
Minimally invasive surgical technique
Positioning of the patient
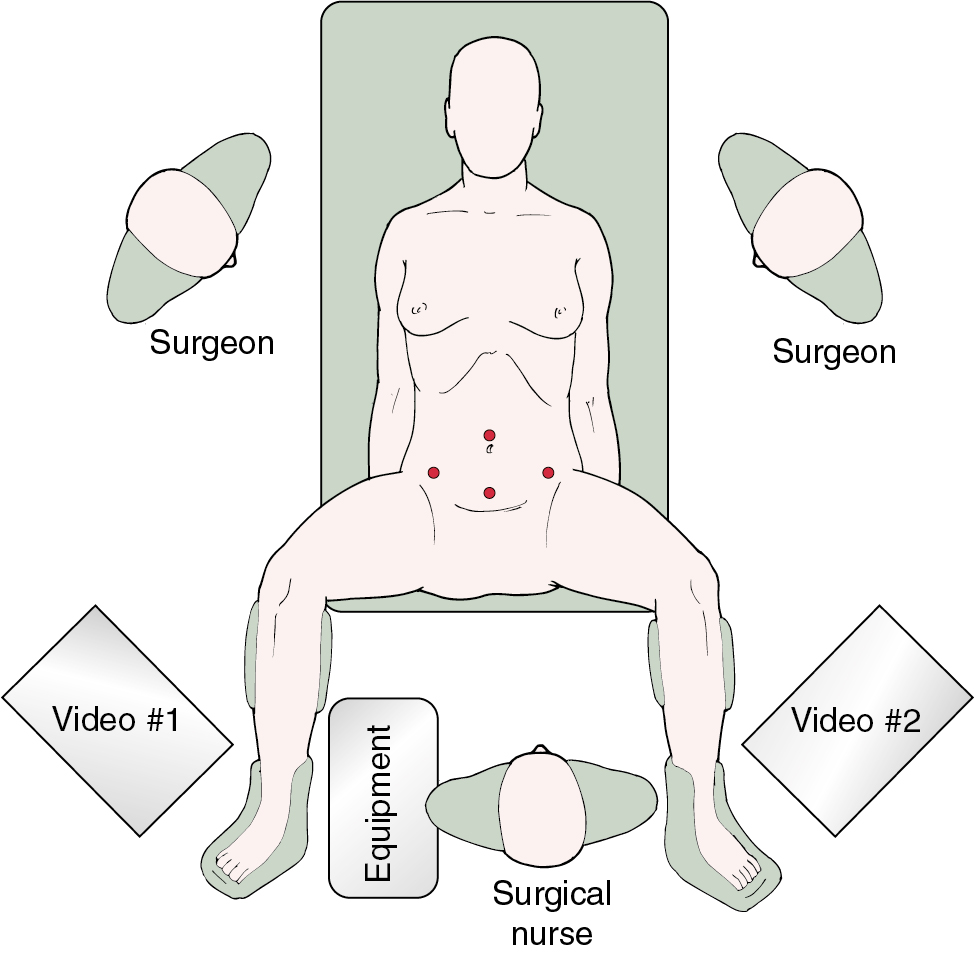
Positioning of the patient is critical in advanced MIS. For most gynecologic procedures, the appropriate position is in a dorsal lithotomy position with adjustable Allen stirrups to allow for manipulation of the uterus and laparoscopic assisted vaginal hysterectomy (LAVH) or total laparoscopic/robotic hysterectomy (TLH) as indicated. In patients who do not have a uterus or in whom the uterus is not anticipated to be removed, placement in a supine position may be appropriate. The patient’s arms should be tucked by her side to allow mobility and ergonomic comfort for the operating surgeon and assistant. Care should be used in protecting both the upper and lower extremities with appropriate padding to prevent pressure points and nerve injuries, and several positioning systems that rely on friction or barrier blocks and straps are available. For laparoscopic surgery, video monitors should be placed on each side of the table across from the operating surgeon and the assistant and located toward the foot of the table. This allows for comfortable positioning of the surgeon in a natural angle of viewing the video monitor and minimizing counterintuitive surgical movement. Placement of the monitors toward the patient’s head can be considered when extensive upper abdominal surgery is undertaken ( Fig. 20.1 ).
Port sites and setup
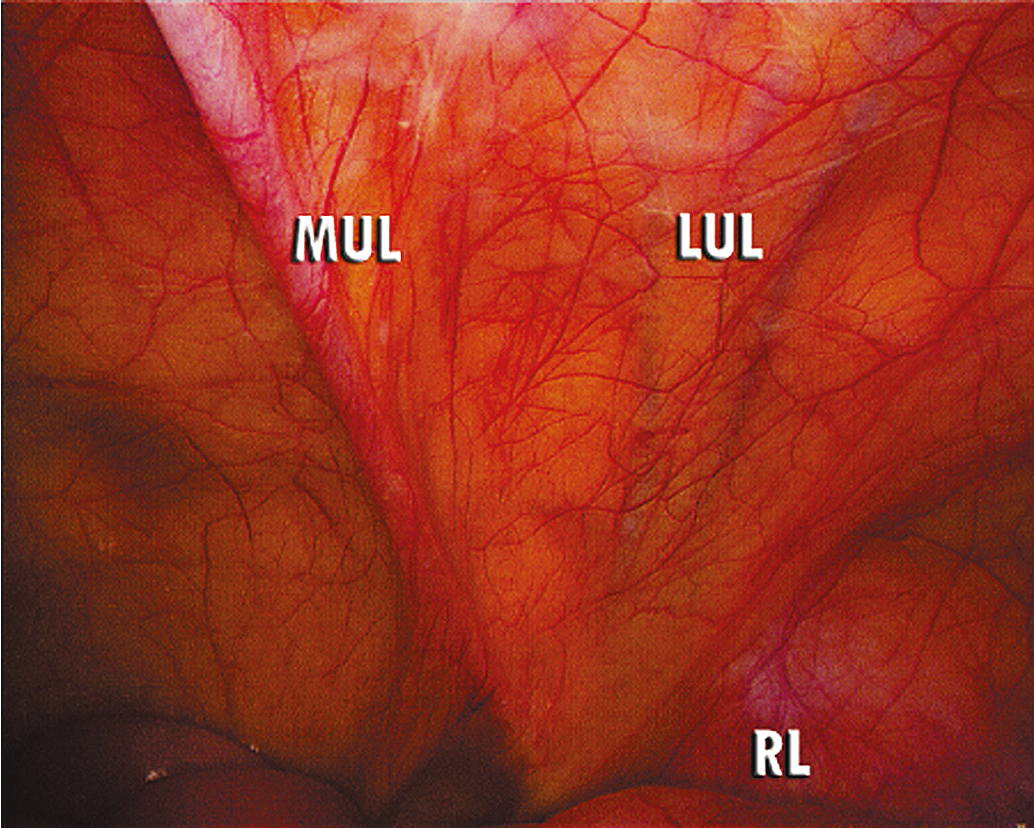
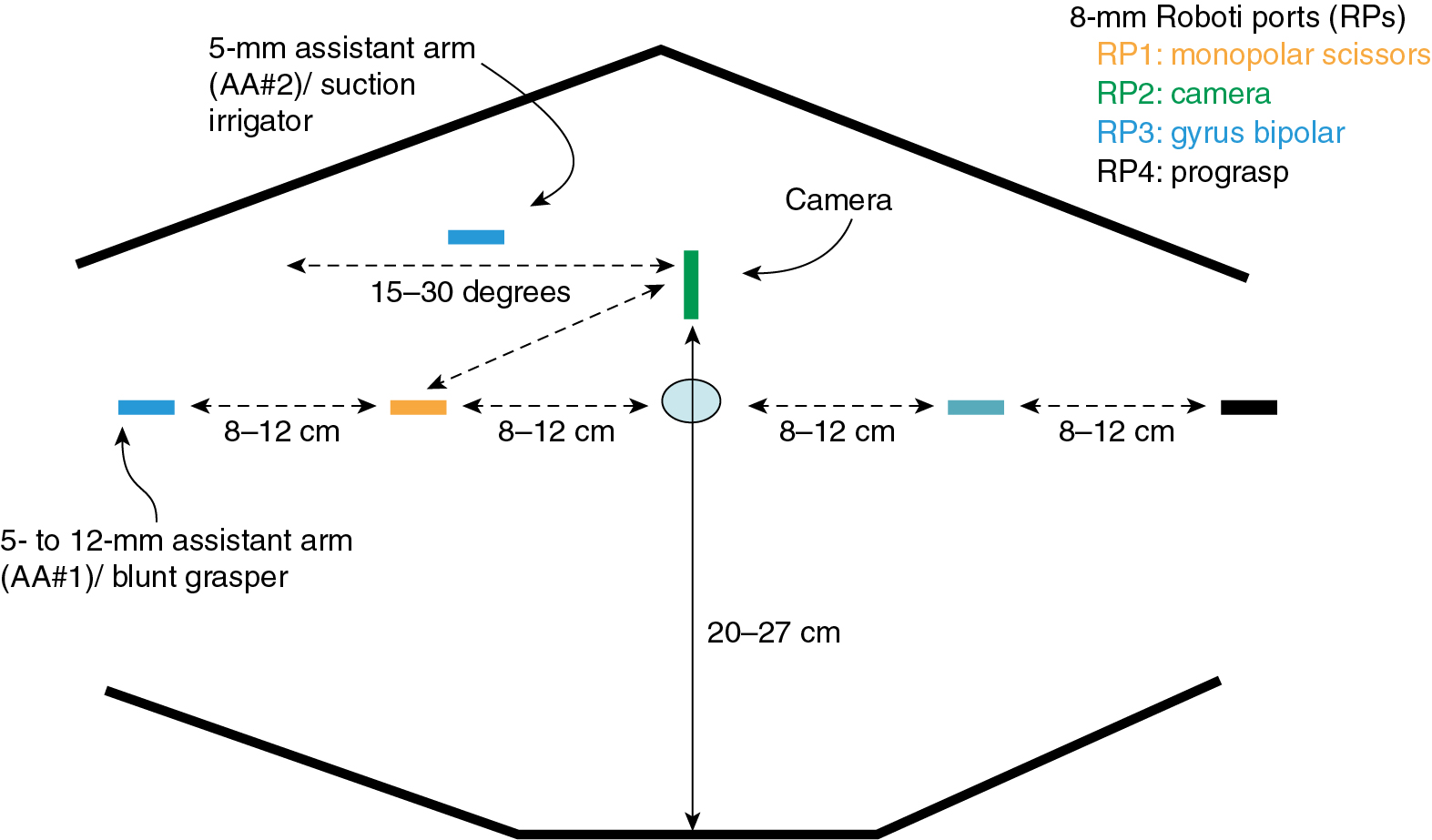
The number, position, and size of trocars for laparoscopic surgery depend on the surgery anticipated. In cases that require removal of an adnexal mass or lymph nodes, a 10- to 12-mm accessory port will be needed for extraction of the specimen. Most LS can be accomplished successfully with the placement of a 5- or 10-mm port at the level of the umbilicus for camera placement, with or without a 10- to 12-mm port suprapubically, and a 5-mm port in each of the lateral lower quadrants. Gynecologic oncologists usually use a total of three to six ports to obtain adequate exposure and accomplish advanced pelvic procedures. Safe placement of the primary port, or camera port, is the most critical part of the procedure in terms of minimizing major surgical complications. A number of surgical approaches have been described and accepted for placement of the primary port. An oropharyngeal tube should be used to achieve gastric decompression before placement of the Veress needle or primary trocar. Lateral ports can safely be placed in a line one-third of the distance from the anterior superior iliac spine to the umbilicus. The oncologist should take care when placing the lateral port and do so under direct visualization with inspection of the deep inferior epigastric vessels lying along the lateral boundary of the rectus abdominis muscles. These can be directly identified lateral to the obliterated umbilical ligaments ( Fig. 20.2 ). Transillumination will not reliably reveal the location of these vessels.
The port-site setup for robotic surgery is different from that for laparoscopic procedures because ports are generally placed above the umbilicus for the SI system and through the umbilicus for the Xi system ( Fig. 20.3 ). With the Xi system’s rotating boom and potential for multi-quadrant surgery, variations in port setup may require moving the camera port while keeping all other robotic ports in the same position during surgery. Various port setups have been described for robotic gynecologic oncology surgery. Most gynecologic oncologists use two or four laparoscopic/robotic ports and one or two additional LS ports to be controlled by the bedside assistant. In addition, the robot must be “docked” or attached to the ports, which is usually accomplished between the legs or from the side of the patient. When the surgical procedure commences, the experienced robotic surgeon can efficiently alternate (swap) control of the various robotic ports through a unique clutching system, using both hand and foot controls can operate all instruments in real time and has direct control of both monopolar and bipolar electrosurgical energy sources. Although there is less reliance on the bedside assistant, that person is still instrumental in facilitating the case through robotic instrument changes, manipulation of vaginal instruments, suction irrigation, and the use of an additional grasping instrument for retraction.
In addition to multiport surgery, the options have expanded to single-port surgery or laparoendoscopic single-site surgery (LESS) and robotic single-site surgery. Single-port (Sp) surgery uses a GelPort or Single Incision Laparoscopic Surgery (SILS) port in a 2- to 3-cm (umbilical) incision. Through this port, the camera port and usually two or three additional trocars are placed. Possible advantages include better cosmesis and decreased postoperative pain. Disadvantages such as port crowding, crossing of instruments, and need for advanced laparoscopic skills have limited the uptake for advanced and complicated pelvic surgeries. Robotic single-site surgery allowed for computerized optical reversal of crossing instruments and hands with improved ergonomics and 3D visualization. An articulated needle driver has been added to the Sp platform, further improving surgeon comfort and easier applicability or single-site surgery. Cases must be carefully selected because Sp will not allow for use in very obese patients with thick subcutaneous tissues, and the use of adequate uterine manipulators is imperative without the availability of ports to provide retraction and manipulation. Sp surgery has been FDA approved for urologic surgery and transoral otolaryngology procedures but is still considered experimental for gynecologic surgery.
Surgery in the overweight and underweight patient
Surgical procedure and technique
After the ports are placed, actual surgical technique varies little except for the surgical steps required to complete various aspects of the procedure. After successful insufflation and placement of the trocars are accomplished, visual inspection of the abdominal cavity is undertaken, and the patient is placed in a steep Trendelenburg position. As in any surgical procedure, excellent exposure should be accomplished initially and maintained throughout the case. Use of steep Trendelenburg position is necessary, in lieu of packing the bowel, to achieve adequate visualization of the pelvis and lower abdominal region. Lysis of any adhesions holding the small bowel or omentum into the pelvis or lower abdomen should occur before beginning the pelvic and upper abdominal dissection. Laparoscopically folding of the bowel into the upper abdomen will improve visualization in the pelvis. The small bowel is carefully placed in the upper abdomen by flipping the bowel up from a caudad to a cranial position, exposing the mesentery of the small bowel and the aortic bifurcation ( Fig. 20.4 ). Blunt instruments and gentle techniques must be used in this maneuver. In obese patients, body habitus may not allow a steep Trendelenburg position because of unacceptably high peak inspiratory pressure. Surgery may be completed by decreasing the amount of Trendelenburg positioning (while the robot is undocked) or by decreasing the insufflation pressure. Adjusting the respiratory frequency and tidal volume may further improve ventilation. In addition, obesity may prevent adequate mobilization of the small bowel out of the pelvis and upper abdomen to allow for retroperitoneal dissection. Use of a 30-degree camera scope may allow for improved visualization in the pelvis in obese patients depending on the port setup and location of the bowel. Some authors have advocated using additional port sites to help circumvent this problem; laparoscopic paddle or fan retractors may also improve exposure. For both obese and underweight patients, using longer laparoscopic and robotic ports may allow for improved manipulation of instruments during the surgery.
In underweight patients, spacing can be maximized once the patient’s abdomen is fully insufflated with carbon dioxide. The use of a left upper quadrant entry at Palmer’s point can allow maximal spacing of the ports along a smaller abdominal wall surface area. The use of longer ports in underweight patients can move the instruments away from each other and minimize potential instrument crossing during the procedure. The camera port, often placed in the midline for oncologic procedures, may need to be shifted away from the midline in underweight patients to ensure adequate spacing of ports. If using a robotic platform, the authors recommend at least 8 cm of spacing between robotic ports for the Si system. This is in contrast to the Xi system, which requires a minimum of 6 cm of spacing between ports to ensure full functionality. For port positioning, in underweight patients, the ports will often need to offset to maximize the distance between ports compared to obese patients where often ports are placed in a straight line.
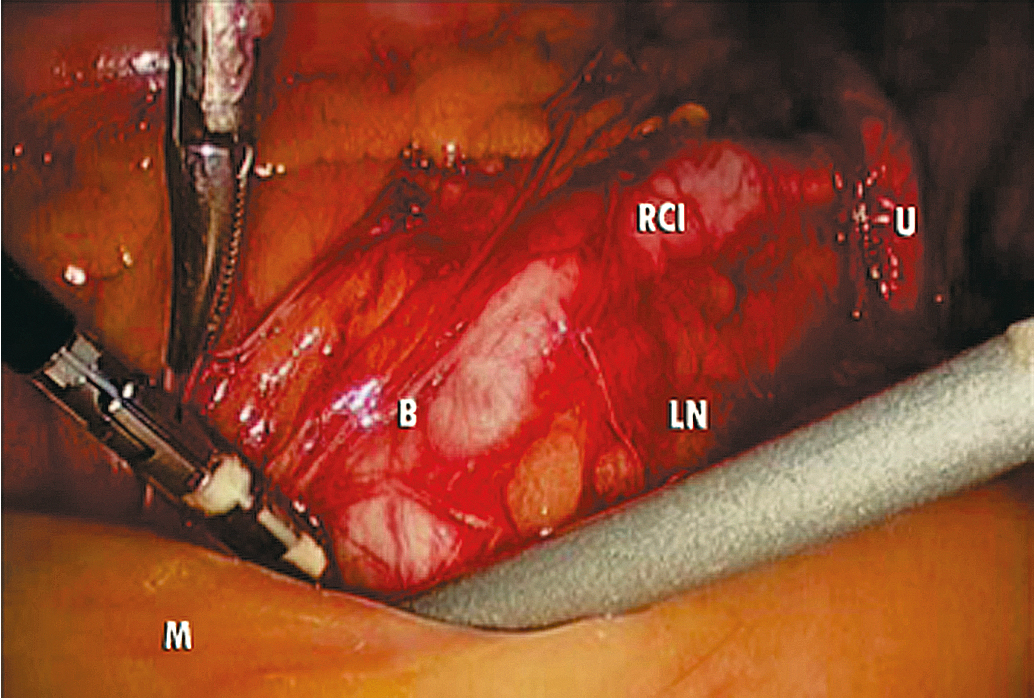
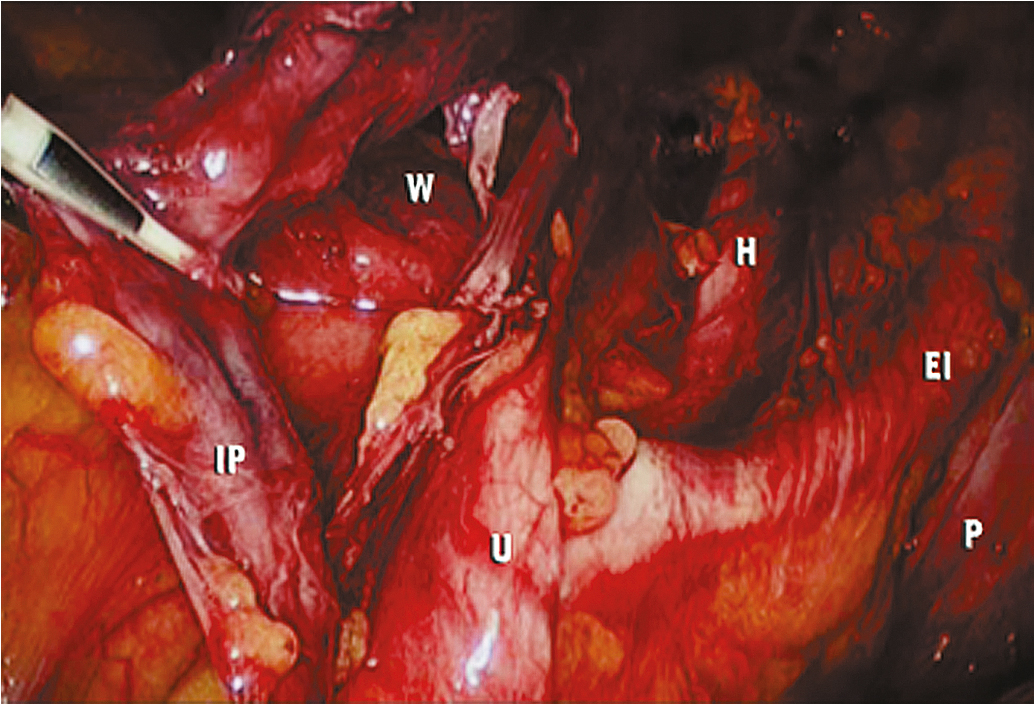
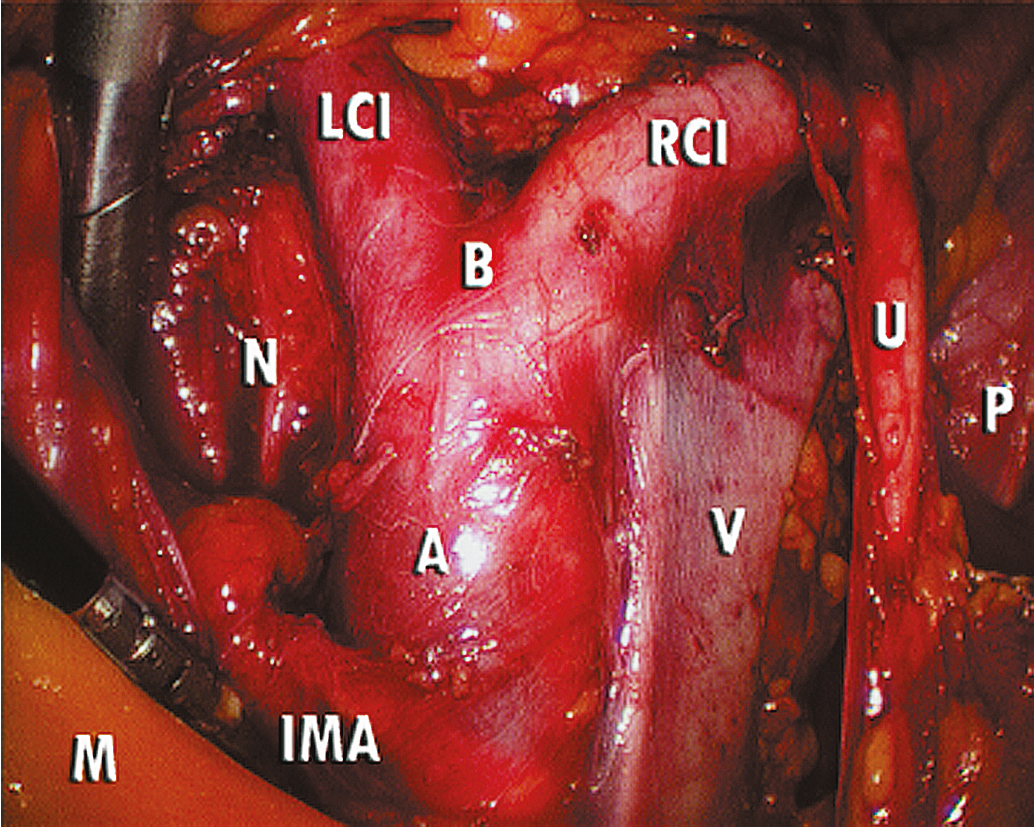
The key to successful advanced MIS is the same as that for open laparotomy: access to the retroperitoneum. In the pelvis, this is accomplished by dividing the round ligament laterally or opening the pelvic peritoneum lateral and parallel to the infundibulopelvic ligament. Some surgeons prefer to keep the round ligament intact so they can retract against it to keep the paravesical space open while dissecting tissue. Dissection is then carried down to the level of the external iliac artery, which is then followed in a cephalad and medial direction to the common iliac artery. At this point, the ureter can be found crossing the pelvic brim, and a window can be created between the ovarian vessels and the ureter ( Fig. 20.5 ). Development of the pararectal space is under direct visualization after identification of the bifurcation of the common iliac vessel and the ureter. The surgeon places traction on the ureter, medially developing the pararectal space between the hypogastric artery laterally and the ureter and rectum medially. Care must be taken during this dissection to avoid disrupting the cardinal web deep in the retroperitoneum. Because of the positive pressure environment of MIS resulting from the pneumoperitoneum, the boundaries of the paravesical space can be identified visually during laparoscopic surgery. The superior vesicle artery and umbilical artery are clearly visible as the medial umbilical ligament (see Fig. 20.2 ). Dissection is carried along the external iliac artery to the level of the superior vesicle artery (obliterated umbilical ligament). At this point, the superior vesicle artery is retracted in a medial direction, and the paravesical space is easily developed with the bladder and superior vesicle artery medially and external artery and obturator node bundle laterally. After this is accomplished, the uterine artery can be clearly identified at the origin of the superior vesicle artery from the hypogastric artery. This retroperitoneal pelvic dissection is the cornerstone of any laparoscopic surgery performed in the pelvis, including removal of an adnexal mass, TLH, LAVH, laparoscopic radical hysterectomy (LRH), and PLN dissection ( Fig. 20.6 ). Dissection can be facilitated with a variety of energy sources and clip appliers, each with its own advocates.
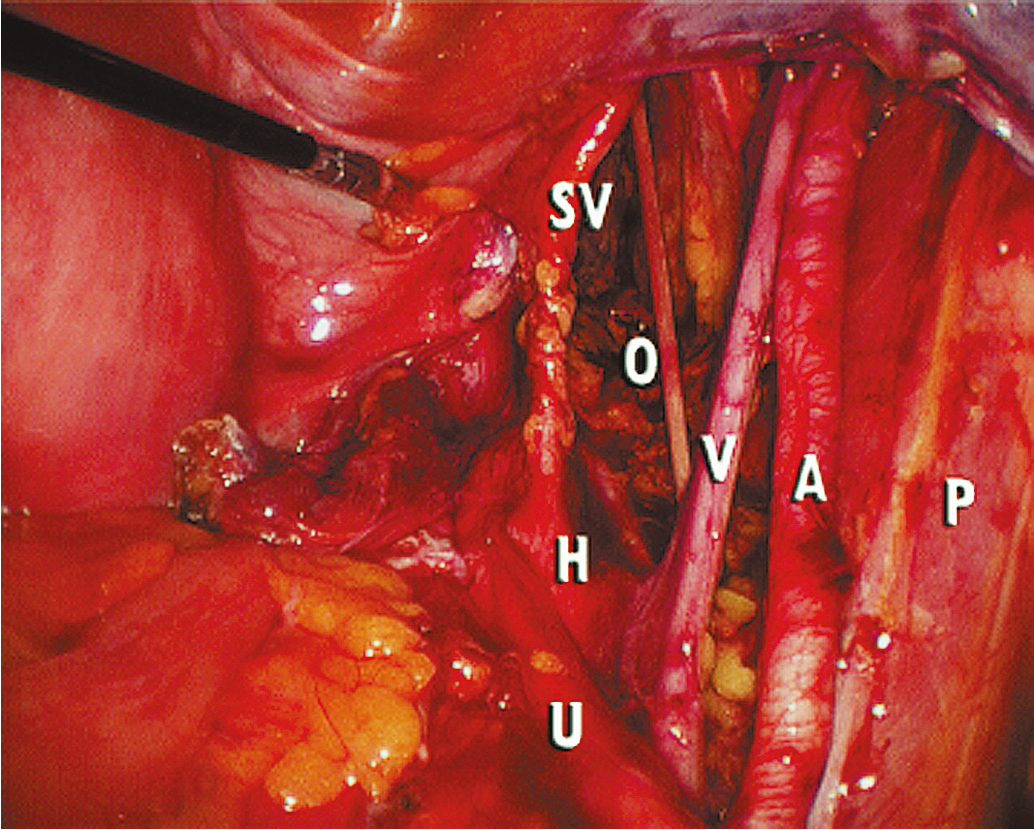
Extension of the incision along the peritoneum overlying the right common iliac artery and then along the aorta to the level of the duodenum allows for exposure of the paraaortic retroperitoneum ( Fig. 20.7 ). During this dissection, the peritoneum attached to the base of the cecum can be elevated in an anterior-cephalad direction, providing excellent exposure to the right paraaortic lymph node region. Margins of resection are identical to an open approach and can be extended to the level of the renal vessels. The left-sided paraaortic lymph node dissection requires dissection underneath the inferior mesenteric artery, mobilizing the descending colon and rectosigmoid off the left common iliac artery and retracting the inferior mesenteric artery and ureter laterally and cephalad. This gives excellent exposure to the left paraaortic lymph nodes inferior to the inferior mesenteric artery. Dissection can be continued above the inferior mesenteric artery in a similar manner. Some authors have advocated sacrificing the inferior mesenteric artery to get enhanced exposure to the upper left paraaortic nodes. Others prefer a extra peritoneal approach with ports placed in the retroperitoneum of the left flank and without entering the peritoneal cavity. Insufflation of the retroperitoneum has the advantage of lifting the bowel and avoiding interference of large and small bowel loops in the field of dissection. Lymph nodes are dissected off the IVC and para aortically in a similar way as via the transperitoneal techniques.
MIS in gynecologic oncology is now common, but especially in the underweight and morbidly obese, attention must be made to patient positioning, port placement, and surgical exposure to ensure patient safety. The surgeon needs to obtain experience over time to optimize patient outcomes and emphasize excellent surgical technique. Knowledge of anatomy, the disease process, and surgical technique is key during complicated surgical procedures. To minimize complications that are possible with any major surgical procedure, vigilance and meticulous surgical technique are required.
Applications of minimally invasive surgery in gynecologic oncology
Cervical cancer
The pioneering reports describing the use of advanced laparoscopic techniques in gynecologic oncology were initially described in patients with cervical cancer in which LS was used for PLN dissection in patients with early-stage disease to assess the feasibility for abdominal radical hysterectomy. One of the initial concerns about MIS in cervical cancer was that the laparoscopic approach would not be as thorough as laparotomy in assessing metastatic cancer and performing a comprehensive lymphadenectomy. Early studies in patients undergoing initial laparoscopic lymphadenectomy before laparotomy confirmed that a thorough pelvic and paraaortic lymphadenectomy is possible via LS, with no positive nodes discovered at laparotomy performed after laparoscopic lymphadenectomy. Once the feasibility of laparoscopic lymphadenectomy was established, the use of MIS for radical surgery in patients with early-stage cervical cancer and for pre-radiation surgical assessment in patients with advanced disease was explored.
Early-stage cervical cancer: Radical hysterectomy
There are various ways in which MIS techniques have been utilized for the management of early-stage cervical cancer ranging from laparoscopic lymphadenectomy with vaginal radical hysterectomy (VRH), laparoscopic-assisted radical vaginal hysterectomy (LAVRH), and total LRH. The Schauta radical vaginal hysterectomy, described more than 100 years ago, and its subsequent modifications are performed completely vaginally; however, the surgeon does not have the opportunity to perform a retroperitoneal lymph node dissection. The LAVRH has many descriptions but usually involves a laparoscopic phase that includes the lymphadenectomy followed by developing the pararectal and paravesical spaces, dividing the parametria and paravaginal pedicles, and completing the remainder of the procedure vaginally. This procedure allows the surgeon to precisely define the vaginal incision and minimize/eliminate tumor exposure to the peritoneal cavity.
The first total LRH was reported in 1992. In this procedure, the entire radical pelvic operation and lymphadenectomy are completed with MIS techniques. The LRH became favored as there is no need for a perineal phase for the performance of a vaginal incision, exposure is improved, visualization of critical anatomy and surgical planes is better, and anatomic limitations are fewer as a result of a narrow pelvis or lack of uterine descensus. Most important, however, is the fact that LRH mimics the well-established steps of an abdominal radical hysterectomy, thereby making LHR easier to adopt.
In 2005, the robotic system was approved by the US FDA for gynecologic surgery, and the first robot-assisted radical hysterectomy (RARH) was described in 2006.
Most surgeons who use robotic techniques feel that the superior vision and wristed instruments provide optimal MIS capacity to perform the meticulous dissection required for the radical hysterectomy. Thus, the availability of the robotic platform allows for a significant shortening of the learning curve for MIS radical pelvic procedures and increased uptake of radical hysterectomy via the minimally invasive approach.
Numerous retrospective reports demonstrate the feasibility and perioperative safety of minimally invasive radical hysterectomy for the management of early-stage cervical cancer ( Table 20.1 ). When comparing LRH and RARH to abdominal radical hysterectomy, MIS reduces overall perioperative complications including blood loss, transfusion, infection, wound complications, pain, and length of stay ( Table 20.2 ). While there is no doubt that the LARVH, LRH, and RARH are feasible for patients with early-stage cervical cancer, its oncologic safety has been challenged.
| Study | Patients (n) | Operating Room Time (h) | Blood Transfusion (%) | Intraoperative Complications (%) | Postoperative Complications (%) | Chronic Genitourinary (%) | Length of Stay (days) |
|---|---|---|---|---|---|---|---|
| Argentina, 1999 | 56 | 4.5 | NR | 2 | 6 | 2 | 4 |
| Quebec, 2000 | 54 | 4.5 | 4 | 7 | 10 | 0 | 5 |
| France, 2002 | 50 | 4.5 | 2 | 4 | 16 | 4 | 8 |
| France 2, 2002 | 95 | 4.0 | NR | NR | 12 | 2 | NR |
| WCC, 2002 | 78 | 3.5 | 1.3 | 9 | 9 | 1.3 | 3 |
| Germany, 2003 | 200 | 5.5 | 19 | 13 | 15 | 3.5 | NR |
| MSKCC, 2003 | 19 | 6.0 | 5 | 11 | 5 | 0 | 4.5 |
| Toronto, 2004 | 71 | 3.5 | 7 | 13 | 14 | 3 | 1 |
| MDA, 2007 | 35 | 5.75 | 11.0 | 11 | >20 | 0 | 2 |
| China, 2007 | 90 | 4.5 | NR | 9 | 40 | 3 | NR |
| Italy, 2009 | 65 | 3.25 | 0 | 5 | 10 | 1.5 | 4 |
| A. Laparoscopic | |||||||
| Reference (year) | Patients ( n ) | EBL (mL) | Operative Time (min) | LN Count | Intraoperative Complications ( n ) | Postoperative Complications ( n ) | Length of Stay (days) |
| 69 | 350 | 236 | 19 | 1 | n/a | 9 | |
| 42 | 145 | 216 | 20 | 1 | n/a | 10 | |
| 46 | 232 | 11 | 0 | 1 | 4.8 | ||
| 50 | 202 | 211 | 23 | 4 | n/a | 8.7 | |
| 76 | 95 | 255 | 38 | 2 | n/a | 4 | |
| B. Robotic | |||||||
| Reference (year) | Patients ( n ) | EBL (mL) | Operative Time (min) | LN Count | Intraoperative Complications ( n ) | Postoperative Complications ( n ) | Length of Stay (days) |
| 60 | 100 | n/a | 18 | 0 | n/a | 11 | |
| 73 | n/a | 152 | 11 | 0 | 3 | 4.1 | |
| 50 | 55 | n/a | 25 | 0 | n/a | 9.6 | |
| 63 | 50 | 213 | 29 | 1 | 2 | 1 | |
| 40 | 78 | 272 | 20 | 2 | n/a | 3.7 | |
| 51 | 96.5 | 248 | 34 | n/a | 4 | 1 | |
| C. Abdominal | |||||||
| Reference (year) | Patients ( n ) | EBL (mL) | Operative Time (min) | LN Count | Intraoperative Complications ( n ) | Postoperative Complications ( n ) | Length of Stay (days) |
| 176 | n/a | 168 | 16 | 1 | 9 | 9.6 | |
| 64 | 400 | 240 | 24 | 1 | 3 | 4 | |
| 40 | 222 | 200 | 26 | 5 | n/a | 5 | |
| 49 | 417 | 211 | 23 | n/a | 8 | 3.2 | |
In 2018, the results from a large, international phase III clinical trial comparing minimally invasive radical hysterectomy to abdominal radical hysterectomy for the management of early-stage cervical cancer were published (Laparoscopic or Robotic Radical Hysterectomy versus Abdominal Hysterectomy in Patients with Early Stage Cervical Cancer [LACC]). This trial randomly assigned women with stage IA1 (+LVSI), IA2, or IBI cervical cancer to minimally invasive radical hysterectomy (laparoscopic or robotic) or open abdominal radical hysterectomy. The trial was suspended early due to an imbalance in deaths between the two groups. At that time, 631 of the planned 740 patients had been enrolled. The rate of disease-free survival at 4.5 years was 86% with MIS and 96.5% with open surgery (difference, -10.6 percentage points, CI -16.4 to -4.7, P = .87 for noninferiority). Thus, noninferiority was not declared. The minimally invasive approach was associated with a lower 3-year disease free (91.2% vs. 97.1%, hazard ratio [HR] 3.74) and overall survival (93.8% vs. 99%, HR 6.0) though these were not prespecified endpoints, and thus P values were not assigned. In this study, average operative time was longer for the MIS group (216 vs 187 min, P < .001); however, EBL was significantly lower (101 vs. 209 mL, P < .001), and length of stay was significantly shorter (3 vs. 5 days, P = .002). The overall rate of intraoperative and postoperative complications was not different between the two groups. A cohort study utilizing national data from the National Cancer Database and the SEER-18 registry was published concurrently with the LACC trial demonstrating similar findings. This study, which analyzed 2461 women (1225 minimally invasive and 1236 open), noted a worsened 4-year mortality among women who underwent MIS compared to those who underwent open surgery (9.1% vs. 5.3%, HR 1.65 [95% CI: 1.22 to 2.22]).
Additional retrospective cohort studies and meta-analyses have been reported since the publication of these landmark studies. While some confirm the findings of the LACC trial, others refute it and/or provide some insight into why the various operative techniques may yield different outcomes. Kim et al utilized the Korean national database to identify women with cervical cancer undergoing radical hysterectomy from 2011 to 2014. To minimize bias, propensity score matching was utilized. In this large cohort study, the authors report lower rates of surgical complications and better overall survival for women undergoing laparoscopic compared to open radical hysterectomy (HR 0.74). These findings contrast with the outcomes reported from the SUCCOR study, an international European cohort observational study comparing MIS to open abdominal radical hysterectomy for patients with IBI cervical cancer. In this study, the risk of disease recurrence and death was more than two-fold higher in women undergoing MIS radical hysterectomy compared to those having surgery via an open approach. Of note, patients who underwent MIS surgery with the use of a uterine manipulator had a 2.76 times higher hazard of relapse (HR 2.76, P < .001), whereas those without the use of a uterine manipulator had a similar disease-free interval to the open surgery group (HR 1.58, P = .2). Additionally, patients who had protective vaginal closure at the time of MIS had similar rates of relapse to those who had an open surgical approach. This study certainly is hypothesis generating as to why differences in outcomes may have been observed in the LACC trial and highlight potentially modifiable factors that can be controlled to improve outcomes with LRH and RARH. Another retrospective cohort study found that while MIS radical hysterectomy was associated with higher rates of recurrence compared to open radical hysterectomy in women with early-stage cervical cancer, PFS was not influenced by surgical approach for women with preoperative tumor size ≤2 cm on preoperative MRI. The LACC trial was not adequately powered to determine if tumor size influenced outcomes with MIS versus open surgical approach, but this certainly is thought provoking.
Most authors stress that there is a lengthy learning curve for LRH and that complications decrease, and overall operative efficiency improves as the surgeon gains experience. This has also been suggested as a possible explanation for the differences in the outcomes observed in the LACC trial and subsequent studies demonstrating increased risk of recurrence and decreased survival with MIS surgical approaches again highlighting the need for additional study.
At this time, open radical hysterectomy is generally considered the preferred approach for women with early-stage cervical cancer (>stage IA2). While oncologic outcomes may be equalized with alterations in surgical technique (i.e., avoidance of uterine manipulators or closure of the vagina prior to colpotomy) or strict selection criteria for patients considered for an MIS approach (i.e., tumor size <2 cm with adequate preoperative imaging), additional study is needed to confirm the safety of LRH and RARH utilizing these strategies. The ROCC trial (NCT04831580) opened in 2022 and will study the safety of robotic radical hysterectomy compared to abdominal radical hysterectomy, incorporating modifications to the procedure as mentioned previously.
Early-stage cervical cancer: Fertility-sparing surgery
The ability to perform an adequate laparoscopic lymphadenectomy combined with the revival of the radical vaginal hysterectomy has led to the development of novel techniques for fertility preservation in young patients with early-stage cervical cancer. The laparoscopic vaginal radical trachelectomy (LVRT) is one of the most exciting applications of MIS in gynecologic oncology. To define the potential number of patients in whom this procedure would be considered, Sonoda and coworkers reported on 435 patients undergoing radical hysterectomy. Eighty-nine of these patients were younger than 40 years of age and had tumors that met the criteria for fertility-sparing radical trachelectomy (which represented 20% of their early-stage population) ( Table 20.3 ). This study clearly shows that there is a substantial population of patients who may benefit from this approach.
|
The LVRT technique combines laparoscopic pelvic and common iliac lymph node dissection with a radical vaginal trachelectomy (with preservation of the uterine fundus and creation of a neocervix). This procedure was initially described by Dargent in 1987; since then, several centers have reported preliminary results on fertility-sparing radical trachelectomy with laparoscopic lymphadenectomy. Survival and fertility follow-up reports have been encouraging. To date, more than 300 cases have been reported with a recurrence rate of 4.1% and a death rate of 2.5%, which falls well within the range of survival seen with traditional radical hysterectomy in similar populations. Randomized prospective comparisons are not available at this time, but case-control studies in matched patients reveal equivalent oncologic outcomes when comparing radical hysterectomy with radical trachelectomy. Plante et al. reported on the obstetric outcomes of 72 patients undergoing the surgery over a 12-year time span. A total of 50 pregnancies occurred in 31 women. Infertility rates and first and second-trimester pregnancy loss did not appear to be increased. Of the patients reaching the third trimester, 22% had preterm delivery (although only 8% were delivered before 32 weeks). There appear to be enough data now published to consider fertility-sparing radical trachelectomy a viable option for select and motivated patients.
Chuang and colleagues first reported robotic fertility-sparing radical trachelectomy in which the entire procedure is performed minimally invasively without a perineal or vaginal phase. Subsequent reports, limited by a small number of patients enrolled, have demonstrated the feasibility of robotic radical trachelectomy with decreased blood loss and length of stay compared with open radical trachelectomy and without compromise of histopathologic or cancer outcomes. Approximately half of the patients who attempted pregnancy were able to conceive, and pregnancy rates were higher in the open surgery group. Whether this is due to shorter follow-up times in the MIS group or a true difference will have to be determined in future studies.
Publication of the LACC trial has called into question the safety of MIS radical trachelectomy. Vieirra et al. conducted a multi-institutional retrospective review comparing outcomes for women undergoing open versus MIS radical trachelectomy for early-stage cervical cancer in women who desired future fertility. There was only one recurrence in the entire cohort, which limits our ability to draw conclusions about the impact of the surgical approach on oncologic outcomes; however, it does suggest these patients have an excellent prognosis regardless of the surgical approach. A large retrospective study utilizing the National Cancer Database evaluated outcomes for women who underwent trachelectomy for stage IA2 to IB1 cervical cancer. In the study period (2010 to 2015), the rate of MIS increased from 29.3% to 75%, and there was no difference in survival based on surgical approach. This study has several limitations based on its retrospective nature and the limited data available in the database chosen. Specifically, we lack information regarding surgeon experience, technique (radical vs. simple), uterine manipulating devices, and colpotomy type (abdominal vs. vaginal). Additionally, we lack data regarding perioperative morbidity, disease-specific outcomes, and reproductive outcomes. It is reassuring, however, that there are no differences in overall survival observed. The international radical trachelectomy assessment (IRTA) study is underway and may help to clarify the impact of the surgical approach on outcomes in this patient population. This study is a collaborative, international, multi-institutional, retrospective study planned to evaluate oncologic outcomes (primary endpoint is disease-free survival) for women undergoing open versus MIS radical trachelectomy.
Advanced-stage cervical cancer: Surgical staging
Patients with advanced-stage disease or bulky early-stage cervical cancers are generally treated with definitive radiotherapy and concurrent chemotherapy. For this group of patients, LS has been used for surgical staging and to assist radiation oncologists in the safe placement of interstitial brachytherapy implants. Even positron emission tomography or computed tomography scans will not identify all patients with extrapelvic disease. Although controversial, surgical staging has been advocated to accurately define the extent of disease and guide the subsequent radiation fields. In patients with gross evidence of lymph node metastasis, their removal has been demonstrated in retrospective studies to improve survival compared with radiation of these nodes without debulking.
Before advanced MIS techniques, retroperitoneal lymphadenectomy for surgical staging of cervical cancer was performed at the time of extraperitoneal laparotomy. This approach afforded the surgeon excellent exposure to both the pelvic and paraaortic nodes with the ability to debulk grossly positive nodes. A transperitoneal laparotomy approach is not recommended because it is associated with a significant increase in the rate of severe postirradiation enteric morbidity compared with extraperitoneal laparotomy, presumably secondary to adhesion formation. Both transperitoneal and extraperitoneal laparoscopic approaches have been described for surgical staging of cervical cancer. Initially, there was concern that transperitoneal laparoscopic lymph node dissection would be associated with increased adhesion formation as experienced with transperitoneal laparotomy for lymphadenectomy. The extraperitoneal laparoscopic approach avoids this risk, but the surgeon only has access to the common iliac and paraaortic lymph nodes and is unable to perform a PLN dissection. However, transperitoneal LS, in general, is associated with fewer intraperitoneal adhesions compared with laparotomy. Blinded studies in animal models reveal a similar rate and severity of adhesions between transperitoneal laparoscopic lymph node dissection and extraperitoneal laparotomy; however, transperitoneal laparoscopic lymphadenectomy is associated with significantly fewer adhesions compared with transperitoneal laparotomy.
Multiple single-institutional series exist describing LS for pretreatment lymphadenectomy for advanced cervical cancer. The procedure is feasible with acceptable morbidity compared with laparotomy. A significant advantage for the laparoscopic approach is avoiding potential complications of a large abdominal incision and quicker postoperative recovery, allowing the patient to proceed to definitive radiation therapy more quickly. Although laparoscopic resection of nodes grossly involved with metastatic disease is technically feasible, it is definitely more difficult compared with laparotomy, especially when the nodes are fixed to surrounding vasculature. Therefore, some surgeons prefer extraperitoneal laparotomy in case they encounter nodes that require debulking. The robotic platform obviates many of the inherent disadvantages of LS. As in LS, the robotic approach can be used for pelvic and paraaortic lymph node dissection. In addition, the advantages of robotic surgery enable the surgeon to perform the more complicated debulking surgeries if necessary.
Endometrial cancer
Laparoscopy
In the population of patients diagnosed with a gynecologic malignancy, MIS is most often applied for those diagnosed with endometrial cancer. Before the availability of advanced laparoscopic procedures, vaginal hysterectomy was a less invasive option for definitive surgical therapy, especially in patients with severe comorbidities and an increased risk of complications secondary to laparotomy. In fact, assuming the disease is confined to the uterus, the curative potential of the hysterectomy should be equivalent regardless of the surgical approach. The disadvantages of the vaginal hysterectomy in endometrial cancer include the inability to fully inspect the peritoneal cavity and retroperitoneum (lymph nodes) for metastatic disease and potential inability to complete bilateral salpingo-oophorectomy (BSO). The addition of LS to the vaginal hysterectomy essentially eliminates these disadvantages. Most patients with endometrial cancer present with apparent early-stage disease. Approximately 15% to 25% will be upstaged as a result of surgical staging. The majority of patients with extrauterine disease have occult (without gross evidence) spread. Current disease assessment modalities such as preoperative imaging, intraoperative palpation, gross inspection, and frozen section of the uterus all are inaccurate compared with comprehensive surgical staging that includes lymph node assessment. Comprehensive surgical staging with lymphadenectomy provides the best definition of the biologic nature of the disease and allows the oncologist to make informed postoperative treatment decisions. Laparoscopic/robotic-assisted vaginal hysterectomy bilateral salpingo-oophorectomy (LAVH-BSO) or total laparoscopic/robotic hysterectomy (TLH) can be substituted for total abdominal hysterectomy bilateral salpingo-oophorectomy (TAH-BSO) in the algorithm presented earlier in this book for the management of endometrial cancer.
The overall management of patients with apparent early-stage endometrial cancer continues to evolve. Endometrial cancer remains the least uniformly managed gynecologic malignancy, even among gynecologic oncologists. It remains controversial whether all patients should have surgical lymph node assessment and, in those undergoing lymphadenectomy, the extent to which lymph node dissection should be performed. Nevertheless, until laparoscopic lymph node dissection was described, comprehensive surgical staging of endometrial cancer by MIS was not feasible. The earliest series published on MIS management of endometrial cancer primarily described pelvic lymphadenectomy with a minimum of patients undergoing a paraaortic lymph node dissection. The ability to perform laparoscopic lymphadenectomy evolved from pelvic nodes to right-sided paraaortic nodes to a bilateral paraaortic lymph node dissection to the level of the inferior mesenteric artery. Reports on surgical staging of endometrial cancer recommend that selected patients at high risk for extrauterine disease have a bilateral para aortic dissection to the level of the renal vessels. This technique has been described laparoscopically but is technically more challenging.
The reported benefits of a laparoscopic approach in endometrial cancer are lower blood loss and transfusion rates, shorter hospital stay, faster postoperative recovery, and superior short-term QoL.
Several prospective randomized trials comparing laparoscopic and open surgery for endometrial cancer staging have been conducted. In 2009, the Gynecologic Oncology Group (GOG) reported the results of the LAP-2 (LS compared with laparotomy for comprehensive surgical staging of uterine cancer: GOG Study) trial, which enrolled 2531 patients with apparent early-stage endometrial cancer. All patients in this study were to undergo complete surgical staging and were randomly assigned in a 2:1 ratio of LS to laparotomy. Of the 1678 patients on the LS arm, 25.8% required conversion to laparotomy (of note, conversion to laparotomy was mandatory if lymph node dissection could not be completed laparoscopically). More than half of these conversions were a result of poor visualization (exposure), 16% were from metastasis, and 11% were from bleeding. Fig. 20.8 graphs the likelihood of converting from LS to laparotomy as a function of body mass index (BMI), age, and evidence of metastatic disease. In this landmark study, there was no significant difference in rate of node positivity between the two groups; however, significantly fewer patients in the LS arm (78.5%) compared with the laparotomy arm (86.4%) had lymph nodes histologically identified from all four primary nodal regions (which include the right and left periaortic and bilateral PLNs). The incidence of intraoperative complications, reoperations, and readmissions was similar, but significantly fewer Common Toxicity Criteria grade II or greater postoperative complications ( P < .0001) occurred on the LS arm (14.3%) compared with the laparotomy arm (21.1%). Similar transfusion rates were seen between the groups (7% to 9%). Consistent with other studies in the literature, operative time was significantly longer for the laparoscopic arm (203 minutes) versus the laparotomy arm (136 minutes). For patients successfully completing laparoscopic surgery, the average length of hospital stay was 2 days versus 4 days for laparotomy ( P < .0001). During the perioperative period, the LAP-2 study revealed significantly better overall QoL, pain scores, resumption of normal activities, and time until return to work in the LS arm. By 6 months after surgery, no significant QoL differences were found except in that of body image. Previously reported but small single-institution studies reveal no differences in survival between LS and laparotomy for the surgical management of endometrial cancer. LAP-2 also examined this most important survival endpoint. The estimated 3-year overall survival rates from this study are 89.8% for LS and 89.9% for laparotomy, with similar rates of recurrence and death from disease. Other international studies such as the Dutch study by Mourits et al. in which patients were randomized to TLH versus abdominal hysterectomy (without lymph node staging) and the Australian study (Laparoscopic Approach to Cancer of the Endometrium [LACE] trial) by Janda and coworkers, which also included pelvic lymphadenectomy reported similar findings. The latter also found better QoL up to 6 months after TLH and with fewer complications.
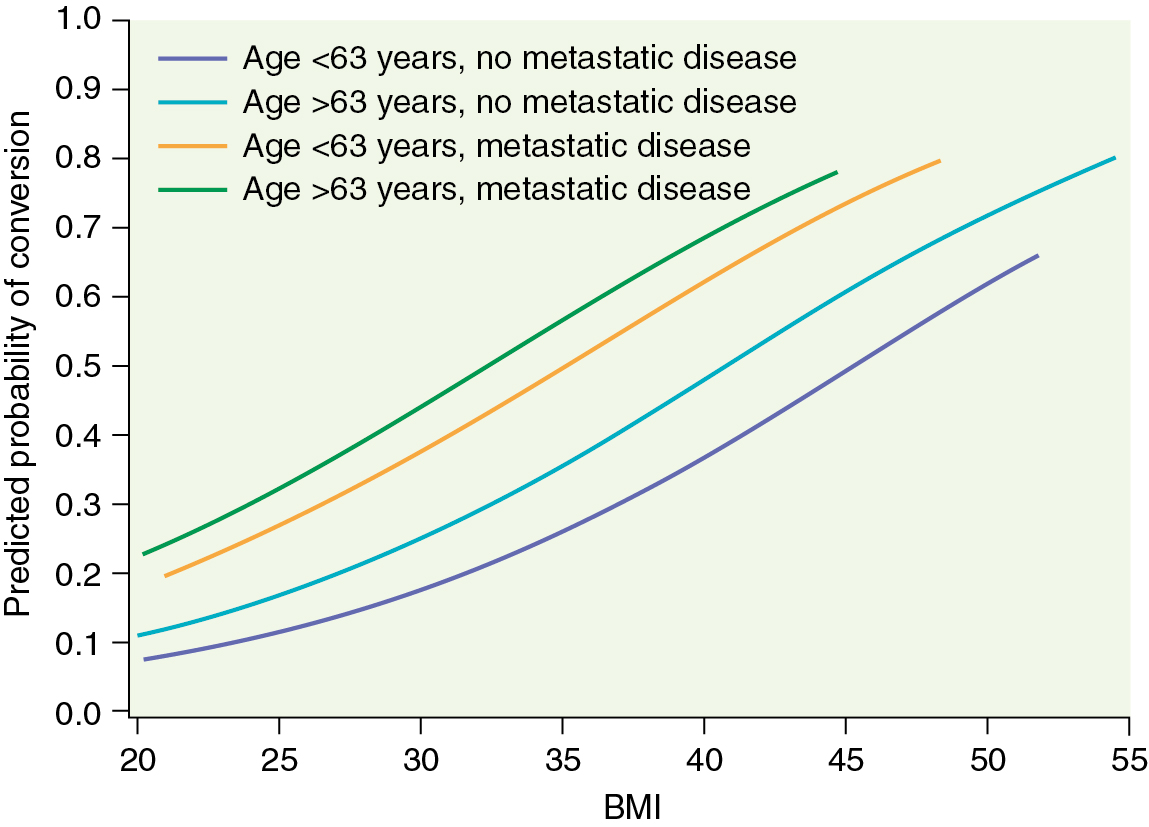
It is now clear that LS offers selected patients with endometrial cancer the potential benefits of shorter hospital stay, less need for blood transfusion, and decreased postoperative complications with a similar risk of intraoperative complications. Nevertheless, laparoscopic hysterectomy with pelvic and paraaortic lymph node dissection remains difficult to learn and is least likely to be offered or successful in patients with obesity, the group of patients most likely to benefit from MIS. Although morbid obesity is the most common risk factor for developing endometrial cancer, it has been a major limitation to successful laparoscopic surgical management. Most studies reporting the use of MIS for the treatment of endometrial cancer describe a population of patients with a mean BMI lower than that typically seen in the overall population of patients with endometrial cancer. Even the LAP-2 study represents a selected group of patients because the median BMI was 28 kg/m 2 in both arms. The rate of conversion from LS to laparotomy ranges quite widely between single-institution reports. Variables such as intent to perform comprehensive staging, adhesions, intraoperative complications, discovery of metastatic disease, and surgeon experience have all been reported as important factors. In the LAP-2 study, the overall conversion rate was 25.8% and strongly influenced by BMI (with a rate of 17.5% in patients with a BMI of 25 kg/m 2 and a rate of 57% in patients with a BMI greater than 40 kg/m 2 ).
Robotics
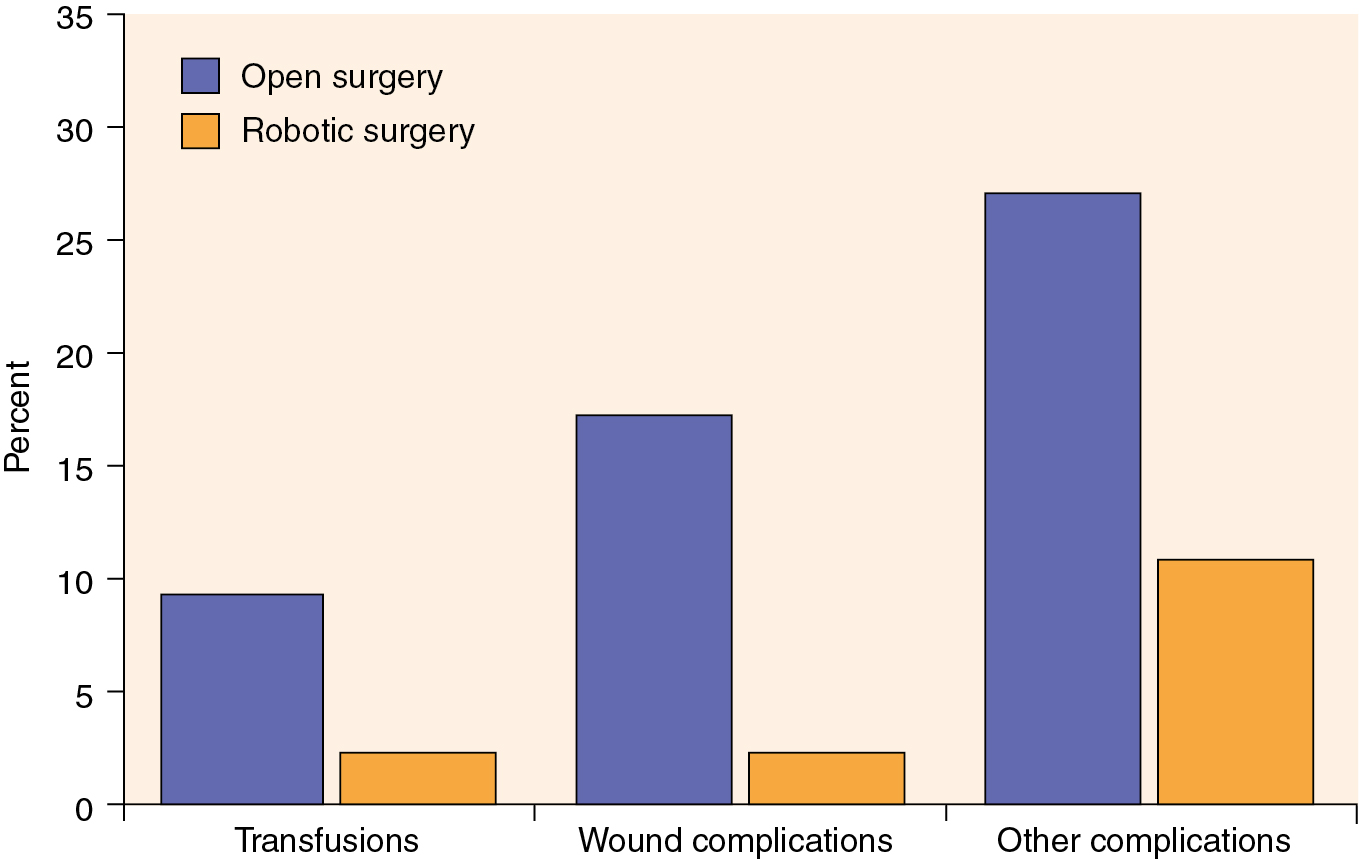
As previously mentioned, developments in robotic technology have allowed the surgeon to gain advantages compared with LS, and this technology has rapidly been adopted by gynecologic oncologists independent of their prior laparoscopic experience. Relatively large single-institution and pooled reports reveal that the robotic surgical staging of patients with endometrial cancer is feasible and that the patient experiences the typical benefits of MIS compared with laparotomy. Furthermore, these reports have shown that robotic surgery is likely applicable to a larger portion of patients diagnosed with endometrial cancer compared with LS, including patients who are morbidly obese ( Table 20.4 ). Seamon and colleagues demonstrated that comprehensive surgical staging is feasible in a relatively heavy group of patients (mean BMI, 34 kg/m 2 ), as robotic pelvic and paraaortic dissection was performed in 85% of the patients overall and in 67% of the patients with BMI more than 45 kg/m 2 . Gehrig and coworkers compared their robotic and laparoscopic experience in obese patients (BMI >30 kg/m 2 ) with endometrial cancer and demonstrated a significant difference in hospital stay, blood loss, and operating room time in favor of the robotic group. Seamon and colleagues compared 105 patients with endometrial cancer undergoing robotic surgery with 76 patients managed laparoscopically and revealed a similar ability to complete surgical staging. However, comprehensive surgical staging was able to be performed in heavier patients compared with LS (BMI, 34 vs. 29 kg/m 2 ), and with significantly shorter length of stay, lower conversion to laparotomy, and shorter operating room time in favor of the robotic group. Compared with laparotomy, the advantages of the robotic approach are more dramatic in obese patients with endometrial cancer. In a matched cohort study in patients with a median BMI of 40 kg/m 2 and multiple comorbidities, patients managed robotically experienced a decreased length of stay (1 vs. 3 days) and significantly lower rates of overall complications (odds ratio [OR], 0.29), blood transfusion (OR, 0.22), and wound complications (OR, 0.10) compared with LS ( Fig. 20.9 ). Even in extreme morbidly obese patients (mean BMIs, 48, 51, and 54 for laparoscopic, robotic, and open hysterectomy, respectively), MIS (laparoscopic and robotic) was associated with lower rates of blood loss and shorter hospital stays despite longer operative times. Similarly, comorbidities, not weight and BMI, were predictive of an increased risk of perioperative complications.
| A. Robotic | ||||||||
| Reference (year) | Patients ( n ) | EBL (mL) | BMI (kg/m 2 ) | Conversion to Laparotomy (%) | LN Count | Intraoperative Complications (%) | Postoperative Complications (%) | Length of Stay (days) |
| 377 | 47 | n/a | 3.5 | 15.5 | 0.5 | 5.9 | 1.4 | |
| 503 | 90 | 32 | 6.4 | 1.6 | 7.6 | 1 | ||
| 103 | 75 | 33 | 3 | 33 | 1 | 5 | 1 | |
| 102 | 109 | n/a | 1 | n/a | 2 | 8.8 | 1.9 | |
| 405 | 88 | 32 | 6.7 | 15.5 | 3.5 | 14.6 | 1.8 | |
| 105 | 100 | 34 | 12 | 31 | n/a | n/a | 1 | |
| 109 | 109 | 40 | 15. | 25 | 2.2 | 11 | 1 | |
| B. Abdominal | ||||||||
| Reference (year) | Patients ( n ) | EBL (mL) | BMI (kg/m 2 ) | LN Count | Intraoperative Complications (%) | Postoperative Complications (%) | Length of Stay (days) | |
| 131 | 198 | NR | 12.1 | 3 | 21 | 5.3 | ||
| 138 | 266 | 35 | 14.9 | 0.7 | 29 | 4.4 | ||
| 131 | 13 | 32 | 13.1 | n/a | 20.6 | 5 | ||
| 191 | 394 | 40 | 24 | 2.5 | 27 | 3 | ||
| C. Laparoscopic | ||||||||
| Reference (year) | Patients ( n ) | EBL (mL) | BMI (kg/m 2 ) | Conversion to Laparotomy | LN Count | Intraoperative Complications (%) | Postoperative Complications (%) | Length of Stay (days) |
| 81 | 146 | 29 | 5% | 23.1 | 3.7 | 10 | 1.2 | |
| 173 | 187 | n/a | 5.2% | n/a | 3.5 | 7.5 | 2.3 | |
| 76 | 250 | 29 | 26% | 33 | 2.6 | n/a | 2 | |
In addition, recurrence rates and survival appear similar to open surgery in retrospective reviews. Although it is most critical that the surgeon have expertise in the management of patients with endometrial cancer, it appears that the learning curve for comprehensive robotic staging of endometrial cancer is not as difficult as LS. Experiences from several institutions have shown that 10 to 20 cases are needed to gain proficiency with the procedure. Operative times improve as the surgeon and the surgical team become more familiar with the setup and techniques. The robotic platform is now the dominant surgical approach in the management of women with apparent early-stage uterine cancer.
Single-site surgery
In 2009, Fader and Escobar reported their initial experience with LESS for gynecologic cancer surgery. Larger series have since been published and have demonstrated safety and feasibility of the single-site approach for endometrial cancer. Although single-site surgery has been reported in moderately obese patients (median BMI, 32 to 33), abdominal wall thickness remains a limiting factor for the SP in extremely morbidly obese patients. Currently, the Sp approach is FDA approved for gynecologic surgeries using the DaVinci Si system, but not the Sp system.
Minimally invasive sentinel lymph node assessment
To reduce the risk of side effects and complications (bleeding, lymphocele, lymphedema, longer operating time) seen with comprehensive lymph node dissection, SLN assessment has been developed and has been part of standard of care in breast cancer, melanoma, and vulvar cancer for several years. SLN assessment is now also an acceptable part of the standard of care in women with endometrial cancer and has been incorporated in the National Comprehensive Cancer Network’s guidelines. SLN assessment can be performed using open and minimally invasive techniques, and equipment is available for laparotomy, laparoscopic, and robotic approaches. Blue dye (isosulfan blue, patent blue, methylene blue) has been studied most extensively. Other available methods include radiolabeled technetium-99 with lymphoscintigraphy, indocyanine green dye (ICG) with immunofluorescence detection, and a combination of the various methods. Several studies advocate for using ICG only given the high detection rates and ease of administration as well as a low risk of allergic reaction (1:40,000). For cervical cancer, peritumoral injection is intuitive and relatively easy. In contrast, the best location of injection is not as clear for endometrial cancer. Investigators have studied hysteroscopic tumor injection, fundal or subserosal injection, cervical injection, or a combination of multiple injection sites. Although combined location injection methods have improved SLN detection rates, the overall accuracy of intracervical injection alone is high. Certainly, some surgeons have expressed concern about missing aortic SLNs, but staging studies have demonstrated that the risk of isolated positive aortic lymph nodes in the setting of negative PLNs is rare (1% to 2%). However, it is important to keep in mind that in high-risk patients (high-grade histology or deep myometrial invasion), the risk of paraaortic lymph node involvement increases to 4%, and as such, the safety of intracervical injection for SLN assessment in high-risk patients has not yet been confirmed. Regardless, it is critically important to visualize all spaces and assess for isolated SLNs. Most surgeons prefer to perform cervical injections only, and injection protocols vary. In general, 1 mL of dye is injected at the 3 and 9 o’clock positions. Some believe dye should be injected superficially (submucosal) and deep (intrastromal), but others inject superficially only. Next, ports are placed, and the retroperitoneal spaces are developed. Care should be taken to maintain excellent hemostasis because blood can obscure visualization of the various types of dye. SLNs are dissected, labeled for location, and sent separately. Detection rates (any detection) vary from 80% to 95% in endometrial cancer and 84% to 99% in cervical cancer, depending on the study and methods used, and best detection in cervical cancer is seen in tumors smaller than 2 cm. Bilateral detection varies between 60% and 85% for cervical cancer and 52% and 82% for endometrial cancer. Adherence to an SLN algorithm is critical for successful application of SLN assessment; all suspicious or enlarged lymph nodes should be removed, and a site-specific complete lymphadenectomy is performed when SLNs are not identified. When adhering to these principles, the negative predictive value is high (95% to 100%), and false-negative rates are low. Most institutions use ultrastaging (serial sectioning of the SLNs with immunohistochemistry in addition to standard hematoxylin and eosin stain) to assess for metastatic disease; however, the clinical significance of isolated tumor cells detected with ultrastaging is still a topic of debate.
Removal of a large uterus
If the uterus is too large or the vaginal opening is too small to remove the uterus through the vagina, the surgeon can place the uterus in a laparoscopic bag (through the vagina or a port site). Sometimes this allows for removal of the uterus through the vagina, providing a smoother outer surface and equal distribution of pulling forces. If still unsuccessful, the midline camera port can be slightly extended to allow for uterine removal in the laparoscopic bag (mini-laparotomy). Some surgeons bivalve or morcellate the uterus in the bag; however, removing the uterus intact for complete pathologic assessment is preferred. Certainly, power morcellation is not recommended when operating on patients with invasive or preinvasive disease.
Uterine manipulation for minimally invasive surgery
Adequate uterine and vaginal manipulation is a requirement for successful completion of MIS to identify the correct planes of dissection and to avoid additional ports to retract and move the uterus. Early studies reported increased incidence of positive peritoneal cytology after laparoscopic hysterectomy (10%) compared with open hysterectomy (2.8%). However, more recently, two prospective studies comparing pelvic washings before and after insertion of the uterine manipulator found no significant increase (0% to 4%) in positive cytology after insertion of the manipulator. Tubal contamination was reported to be higher after robotic compared with laparoscopic hysterectomy, and avoiding manipulation of the tubes but rather retracting using the round ligaments may be considered. There has been no evidence that true lymphovascular invasion is more common with the use of uterine manipulators in endometrial or cervical cancer. Most importantly, there is no evidence that the use of a uterine manipulator leads to increased risk of recurrence or worse prognosis, although randomized studies have not been performed.
Adnexal mass
One of the most common clinical scenarios presented to gynecologic surgeons is that of the suspicious adnexal mass. Because most masses are asymptomatic, the major reason for surgical removal is to determine if a malignancy is present. The finding of a persistent ovarian mass represents a major reason for surgery in gynecology. Killackey and Neuwirth reported that 17% of laparotomies in gynecology are performed primarily for this indication. It has been reported that 5% of persistent premenopausal ovarian masses and 20% to 50% of those in postmenopausal women are malignant. This risk of malignancy (and potential need for surgical staging or cytoreduction) has led to the recommendation that suspicious ovarian masses be removed through a vertical midline incision. This recommendation, however, is made with the assumption that a gynecologic oncologist or other trained surgeon will be available to provide surgical support if required. This surgery may include radical pelvic or upper abdominal dissection, retroperitoneal lymphadenectomy or lymph node debulking, or bowel resection. Most gynecologists do not have such assistance on standby for every surgery performed for a pelvic mass. Potential advantages of minimally invasive approaches to the pelvic masses are cost savings and decreased morbidity in those women without cancer and early diagnosis and referral for appropriate surgical management in those who are found to have malignancies.
Postmenopausal women undergoing laparotomy for ovarian masses have a much greater likelihood of malignancy than premenopausal women. Postmenopausal patients with ovarian masses differ from premenopausal patients with respect to the predictive value of CA-125. An elevated CA-125 level has a positive predictive value for malignancy of 80% to 98% in this population. Postmenopausal patients with an adnexal mass and an elevated CA-125 level are presumed to have a malignancy and should have surgery by an ovarian cancer specialist regardless of the ultrasound findings. The ability to identify patients preoperatively at highest risk for cancer (with the appropriate selection of surgical approach and consultation or referral for surgical management of this potential cancer) is often challenging. Although pelvic ultrasonography, tumor markers, and clinical presentation all contribute to risk stratification for ovarian cancer, the definitive evaluation of a mass is determined during surgery. However, multiple scoring systems have been devised to provide the surgeon with a preoperative probability of a mass being malignant. The SGO and the American College of Obstetrics and Gynecology jointly published referral guidelines and management recommendations for patients who present with a pelvic mass. Chapter 8 includes a comprehensive discussion of risk assessment of an adnexal mass in both premenopausal and postmenopausal women. In general, if a surgeon stratifies the risk of an adnexal mass into low-, medium-, and high-risk categories for malignancy, then the risk of finding an “unexpected” ovarian cancer at the time of surgery should be extremely low.
Minimally invasive surgery management
Several authors have reported on the LS management of suspected benign ovarian masses. In 1992, Nezhat et al. reported 1209 adnexal masses managed laparoscopically. The majority of patients had endometriosis or functional cysts. However, 64 patients had benign ovarian tumors, and four were malignant. There were no reported major complications in removing masses up to 25 cm in diameter, clearly demonstrating the technical feasibility of managing ovarian masses laparoscopically. Since then, multiple authors have reported on the use of laparoscopic oophorectomy in both premenopausal and postmenopausal women. The complication rates in these nonrandomized reviews of laparoscopic surgery vary from 0% to 18% and include bowel injury, ureteral injury, wound infection, hematoma, and hemorrhage. A consensus of these retrospective reviews and small randomized trials is that laparoscopic management of adnexal masses is associated with decreased or similar operating time and decreased perioperative morbidity, including pain, infection, and blood loss compared with laparotomy. These studies also show a decreased length of stay and potential cost savings; however, major complications are still possible.
More recently, authors have reported on using LS in the initial management of ovarian masses suspicious for malignancy. Dottino and coworkers reported on 160 patients with suspicious adnexal masses who had no evidence of gross metastases or extension above the umbilicus. No distinction was made based on other risk factors for malignancy; however, all of these patients were referred for gynecologic oncology consultation. A total of 141 patients were successfully managed laparoscopically. Invasive ovarian cancer was discovered in nine patients, borderline ovarian tumors in eight, and nongynecologic cancer in four. Dottino and associates reported a 3% incidence of intraoperative complications requiring conversion to laparotomy and only one incidence of intraoperative spillage of tumor. This was a sex cord–stromal tumor, which did recur locally. Canis et al. reported on 230 adnexal masses suspicious or solid at ultrasound examination evaluated initially by LS. Twenty percent of the invasive cancers, and 50% of the borderline tumors had cyst puncture or rupture at the time of diagnosis. One case of tumor dissemination occurred with morcellation of an immature teratoma. These studies highlight the need to prevent tumor spill and morcellation for all suspicious masses. There is concern that the positive-pressure carbon dioxide environment established during pneumoperitoneum may predispose patients to intraperitoneal seeding. Animal studies have shown an increased seeding rate in the pneumoperitoneum group compared with control participants. This may be explained by peritoneal damage and exposure of the underlying basal lamina, which could facilitate implantation. No clear conclusions can be drawn regarding the risk to humans, but these studies suggest cyst rupture or spillage should be avoided in all ovarian masses that could possibly be malignant.
The algorithm presented in Fig. 20.10 is based on a strategy of maximal use of MIS combined with minimal risk of unexpected finding of ovarian cancer or intraabdominal tumor spill. The presence of a unilocular cystic mass in premenopausal women is rarely malignant regardless of size. This fact is important because most of these patients are candidates for ovarian conservation with cystectomy. The same criteria for conservative management can be used with MIS as is already being done with laparotomy. Cystectomy has been described using a variety of techniques using MIS. Equipment such as needle aspirators and intraabdominal bagging devices can allow for cyst decompression without spillage. Techniques have been described for either transabdominal or transvaginal cyst aspiration and removal. Large cysts can be aspirated transvaginally and removed via a posterior colpotomy with no intraabdominal spillage. Size limits for removal of unilocular cysts seem to be related to safety of trocar insertion, surgical exposure, and experience of the surgeon. If oophorectomy is to be performed, the aspiration of the unilocular cyst will allow most of these to be removed through the anterior abdominal wall trocar site. More complex cysts that are believed to be benign can be removed in a bag through a colpotomy or by extending the midline port-site incision at the end of the case. Some multilocular ovarian neoplasms larger than 8 cm in size that are thought to be benign based on ultrasonography have a large dominant cyst. These can be managed through a posterior colpotomy with transvaginal drainage of the dominant cyst and subsequent removal of the ovary through the colpotomy. Suspicious masses should not be intentionally aspirated or morcellated outside of a controlled situation that prevents tumor spill and intraperitoneal dissemination.