Outline
Laparoscopic Surgery in Gynecologic Oncology
Laparoscopic Surgical Staging of Gynecologic Malignancies
Robotic Surgery in Gynecologic Malignancies
Minimally Invasive Surgery Learning Curve
Minimally Invasive Surgical Technique
Applications of Minimally Invasive Surgery in Gynecologic Oncology
Complications of Laparoscopic Surgery
Key Points
- 1.
Minimally invasive surgery (MIS) is practiced by more than 90% of gynecologic oncologists.
- 2.
Knowledge of anatomy, the disease process, and surgical technique is key during these complicated surgical procedures.
- 3.
Several studies have shown that 10 to 20 cases are needed to gain proficiency with a certain procedure.
- 4.
MIS reduces blood loss, transfusions, length of hospital stay, and wound complications without compromising adequacy of the procedure or staging even in (extremely) morbidly obese patients.
- 5.
Survival outcomes for endometrial and cervical cancer are similar after MIS and laparotomy.
- 6.
Several safe methods exist to extract an enlarged uterus after MIS. Morcellation is not recommended when there is suspicion or proven preinvasive or invasive disease.
- 7.
Laparoscopy can be used in ovarian cancer to assess the extent of disease and chance of complete debulking surgery. MIS in advanced or recurrent ovarian cancer is still under investigation, and prospective trials are ongoing.
Laparoscopic Surgery in Gynecologic Oncology
Recent advances in the techniques of minimally invasive surgery (MIS) have greatly expanded its role in the management of gynecologic malignancies. Before the 1990s, MIS was mostly limited to laparoscopy for diagnosis of pelvic disease and for tubal sterilization procedures. The great majority of gynecologic oncology procedures for definitive surgical management were performed via large midline abdominal incisions to accomplish appropriate extirpation of the malignancy and surgical staging. Laparotomy causes significant trauma to the patient with many potential associated morbidities, which are increased in incidence and severity in patients with comorbid conditions. The potential applications for MIS in patients diagnosed with gynecologic malignancies have gradually expanded over the past 20 years with improvements in video-laparoscopic instrumentation and surgical training. Since 2005, advances in robotic surgery have led to the increased use of MIS for comprehensive surgical management of patients with gynecologic cancers. Most gynecologic oncologists now offer MIS as an option for surgical management of patients diagnosed with cervical, endometrial, and ovarian neoplasia.
Advanced laparoscopic procedures have been an option for a subpopulation of gynecologic oncology patients in the 1990s. The goal of MIS is to decrease patient discomfort, hospital stay, and short- and long-term morbidity while at the same time providing an overall improvement in quality of life (QoL) and allowing for earlier implementation of other adjuvant therapies if necessary. Incorporating laparoscopic procedures into the comprehensive management of gynecologic oncology patients has been only moderately successful secondary to limitations of the available technology; a difficult and long learning curve; variability in surgeon training and experience; longer operative times; and patient factors limiting the use of MIS such as prior surgeries, extent of disease, and obesity. Technologic limitations to rapid implementation of complicated laparoscopic surgery in gynecologic oncology include a limited two-dimensional field of vision, counterintuitive motions often required for the surgeon and assistant, limited degrees of freedom of the instruments, and ergonomic disadvantages for the surgeon. Advanced robotic technologies have improved most of these limitations, allowing for expansion of the role of MIS in patients with gynecologic cancer.
As illustrated in other chapters in this textbook, there is a long history in the evolution of surgery in patients with gynecologic cancers. These include incorporation of modifications to radical surgical procedures that reduce the toxicities of treatment with the goal of obtaining the optimal oncologic outcome while improving overall patient QoL. It is important that the goals of cancer control and patient safety are not compromised by a given surgical approach. In other words, MIS serves as another tool to achieve these goals rather than a separate end itself. The historical advantages of laparotomy compared to laparoscopy include maximal surgical exposure, three-dimensional (3D) vision, direct tissue palpation and manipulation, and ease of suturing and other instrument use. Improvements in MIS technology, such as the robotic platform and 3D laparoscopy, have allowed MIS to get a step closer to the surgical experience with laparotomy. The previously mentioned disadvantages of MIS are balanced by potential patient outcome–related improvements. Regardless of surgical approach, it is still imperative that the surgeon adheres to the primary surgical principles of optimal exposure, meticulous tissue dissection, expert knowledge of anatomy, and an understanding of the natural history of the diseases being treated to overcome any potential compromise.
Laparoscopic Surgical Staging of Gynecologic Malignancies
The cornerstone of appropriate surgical staging is an accurate pelvic and paraaortic lymph node dissection. Incorporation of MIS into the surgical management of patients with gynecologic cancer was not feasible until a technique for adequate laparoscopic lymph node dissection was made possible. Pioneering descriptions of laparoscopic procedures for pelvic lymph node (PLN) dissection came from Europe in the late 1980s by Dargent and Querleu and coworkers. Although exciting in terms of the prospects of MIS, there were concerns regarding the adequacy of lymph node dissection, operative risks, and ability to access the paraaortic nodes. Since that time, laparoscopic skills evolved, and this approach has been demonstrated to be feasible with many descriptions of the surgical technique and outcomes of the laparoscopic pelvic and paraaortic lymph node dissections now reported. Data concerning the laparoscopic approach in the surgical treatment of gynecologic oncology patients are primarily nonrandomized and retrospective, aiming to establish the feasibility of MIS lymphadenectomy in highly selected patients. Only a few multicenter prospective randomized clinical trials have been completed regarding the feasibility of comprehensive laparoscopic management in patients with gynecologic cancer. These trials have demonstrated that laparoscopy does not have inferior outcomes compared with laparotomy in terms of surgical procedures, adequacy of staging, complications, and survival.
Robotic Surgery in Gynecologic Malignancies
Robotic surgery overcomes many of the technologic limitations of laparoscopy. The robotic platform provides the surgeon with superior high-definition 3D vision, magnification, wristed instruments, and motion scaling. The surgeon has much more ability to directly control the operative field compared with traditional laparoscopy, thus eliminating many important disadvantages of laparoscopy. The operator of the robotic platform not only has improved vision but also controls the direction and distance of the camera from the operative field without relying on the assistant. In addition, the surgeon has three other port sites to use for a dissector, cutting instrument, and another retracting instrument. Loss of haptic feedback is a potential disadvantage; however, the experienced surgeon is able to overcome loss of this sense with heightened visual feedback and meticulous surgical technique. In addition, there are no significant ergonomic disadvantages and a reduced risk of injury to the surgeon and much less risk of fatigue has been demonstrated in some studies. These significant technological improvements have allowed the gynecologic surgeon to perform much more complicated surgeries via MIS on a heterogeneous group of patients. Ten years after Food and Drug Administration approval of robotic surgery for gynecologic surgery (2005), many publications have confirmed the safety and feasibility of robotic surgery compared with traditional laparoscopy and laparotomy. Initial series focused mostly on staging procedures for endometrial cancer; however, the experience has evolved to more advanced procedures such as radical hysterectomy, radical trachelectomy, and even ovarian cancer debulking surgery and pelvic exenteration. It is now the dominant MIS surgical approach in gynecologic oncology
Minimally Invasive Surgery Learning Curve
Recent advances in the techniques of MIS have greatly expanded its role in the management of gynecologic malignancies. Several recent studies have shown an increasing use of laparoscopy as a treatment modality in gynecologic malignancies. Although advanced laparoscopic technology has been available for more than 2 decades, the incorporation of MIS into surgical management of the gynecologic oncology patient has been relatively slow. Whether this relative lack of mainstream adaptation of LS into the practice is a result of technology or training limitations is unclear, although strong biases support both potential causes. Although difficult to quantify, there is a long and difficult learning curve related to laparoscopy and advanced pelvic operations. The availability of the robotic surgery platform is rapidly expanding the role and adaptation of MIS in the surgical management of women with gynecologic cancer. Nevertheless, these procedures remain major surgeries performed through small incisions and therefore offer unique challenges to the surgeon. Appropriate patient selection, along with a comprehensive understanding of surgical techniques and contraindications, allows for optimal utilization of MIS in the gynecologic oncology patient population.
Little information exists to define the learning curve for laparoscopy and gynecologic oncology surgical procedures. Eltabbakh reported on 75 consecutive patients for laparoscopic management of women with endometrial cancer undergoing laparoscopic-assisted vaginal hysterectomy (LAVH), bilateral salpingo-oophorectomy (BSO), and PLN sampling. There was both a significant decrease in operating time and a significant increase in the number of PLNs harvested with increasing surgeon experience. The experience of the operative assistant has also been shown to be important in major laparoscopic procedures for gynecologic malignancies. Scribner and associates reported a significant difference in successful completion of laparoscopic lymphadenectomies when the first assistant was a skilled attending surgeon compared with a resident or fellow in training. The primary surgeon’s experience is the most important factor in improving outcomes in gynecologic oncology laparoscopic surgery. Melendez and colleagues noted improvements in operating room time and a decrease in complications over time in patients with endometrial cancer; however, it required 12 years to gain this experience in 124 patients with endometrial cancer, which reflects a highly selected population of patients. Based on this report and others, it likely requires roughly 20 to 25 laparoscopic endometrial cancer cases to gain proficiency in this procedure, but individual surgeon experience and outcomes are quite heterogeneous. In the largest single-institution report on transperitoneal laparoscopic pelvic and paraaortic lymph node dissection, Schneider and colleagues estimated 20 operations were required to gain the needed experience for laparoscopic pelvic lymphadenectomy and up to 100 for paraaortic lymphadenectomy.
The robotic platform is being more rapidly adapted by gynecologic oncologists to perform extrafascial and radical hysterectomies with pelvic and paraaortic lymph node dissections and is similarly associated with a learning curve. Seamon and colleagues determined that proficiency for hysterectomy with pelvic and paraaortic lymph node dissection in women with endometrial cancer is achieved at 20 cases, and further efficiency continues to improve over time. Holloway and colleagues demonstrated improved operating room times and ability to perform aortic node dissections in a similar group of patients with improvement in patient safety over time. The learning curve for the robotic radical hysterectomy has not been defined but likely is longer than the extrafascial hysterectomy because this is a more complicated surgical procedure. Patient selection based on previous surgical history and, most important, level of obesity significantly influence the learning curve (in addition to surgeon experience in both laparoscopic and robotic MIS.)
The laparoscopic surgical technique is principally different from open laparotomy in the loss of 3D vision, counterintuitive movements, subtle tactile sensation, limitations of instrumentation, and a heavy reliance on skilled surgical assistance. The surgeon needs to compensate for these losses with a thorough understanding of abdominal and pelvic anatomy when operating laparoscopically. In a prospective, randomized trial, Coleman and Muller reported significant improvement in laparoscopic proficiency in residents exposed to a laboratory-based skills curriculum. Training specifically geared toward laparoscopic surgery using models, cadavers, and animal laboratories is important in gaining proficiency in advanced laparoscopic surgery. The learning curve for robotics does appear to be distinctly different from that for laparoscopy as investigators have demonstrated that surgical drills and suturing are performed with enhanced precision and dexterity when comparing robotic technologies with laparoscopy in a training laboratory. In a 2002 survey of laparoscopy training among Society of Gynecologic Oncology (SGO) members, 85% reported receiving no or limited laparoscopic training during their fellowship. A follow-up survey in 2009 among members of the SGO demonstrated that 91% performed laparoscopic surgery in their practices even though 76% reported that they had limited or no exposure to laparoscopic training during fellowship. Ninety-seven percent of gynecologic oncologists now perform robotic surgery. The introduction of simulators, formal resident and fellow training, and the dual-console da Vinci system (Intuitive Surgical) in 2009 allow for a safe training environment and similar patient outcomes compared with traditional laparoscopic surgery. A recent survey of gynecologic oncology fellows showed that most fellows considered even advanced pelvic procedures such as radical hysterectomy and trachelectomy appropriate for MIS. Sixty percent of fellows performed at least 11 procedures per month, an increase compared with 2007. The transition from laparoscopy to robotic surgery for the MIS management of gynecologic cancers will likely further diminish experience in laparoscopic surgery in this population. There may be an advantage to the robotic platform for novice minimally invasive surgeons given the ability for higher magnification and 3D vision. However, any novel technology does not obviate the need for sound surgical principles and technique, knowledge of anatomy, and understanding of the natural history of the diseases being treated.
Minimally Invasive Surgical Technique
Positioning of the Patient
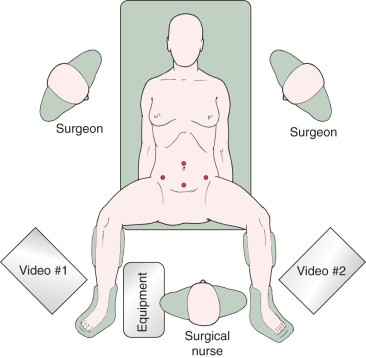
Positioning of the patient is critical in advanced MIS For most gynecologic procedures, the appropriate position is in a dorsal lithotomy position with adjustable Allen stirrups to allow for manipulation of the uterus and LAVH as indicated. In patients who do not have a uterus or in whom the uterus is not anticipated to be removed, placement in a supine position may be appropriate. The patient’s arms should be tucked by her side to allow mobility and ergonomic comfort for the operating surgeon and assistant. Care should be used in protecting both the upper and lower extremities with appropriate padding to prevent pressure points and nerve injuries. For laparoscopic surgery, video monitors should be placed on each side of the table across from the operating surgeon and the assistant and located toward the foot of the table. This allows for comfortable positioning of the surgeon in a natural angle of viewing the video monitor and minimizing of counterintuitive surgical movement. Placement of the monitors toward the patient’s head can be considered when extensive upper abdominal surgery will be undertaken ( Fig. 21.1 ).
Port Sites and Setup
The number, position, and size of trocars for laparoscopic surgery depend on the surgery anticipated. In cases that require removal of an adnexal mass or lymph nodes, a 10- to 12-mm accessory port will be needed for extraction of the specimen. Most laparoscopy (LS) can be accomplished successfully with the placement of a 5- or 10-mm port at the level of the umbilicus for camera placement, a 10- to 12-mm port suprapubically, and a 5-mm port in each of the lateral lower quadrants. Gynecologic oncologists usually use a total of four to six ports to obtain adequate exposure and accomplish advanced pelvic procedures. Safe placement of the primary port, or camera port, is the most critical part of the procedure in terms of minimizing major surgical complications. A number of surgical approaches have been described and accepted for placement of the primary port. An oropharyngeal tube should be used to achieve gastric decompression before placement of the Veress needle or primary trochar. Lateral ports can safely be placed in a line one-third of the distance from the anterior superior iliac spine to the umbilicus. Care should be taken when placing the lateral port and to do so under direct visualization with inspection of the deep inferior epigastric vessels lying along the lateral boundary of the rectus abdominis muscles. These can be directly identified lateral to the obliterated umbilical ligaments ( Fig. 21.2 ). Transillumination will not reliably reveal the location of these vessels.
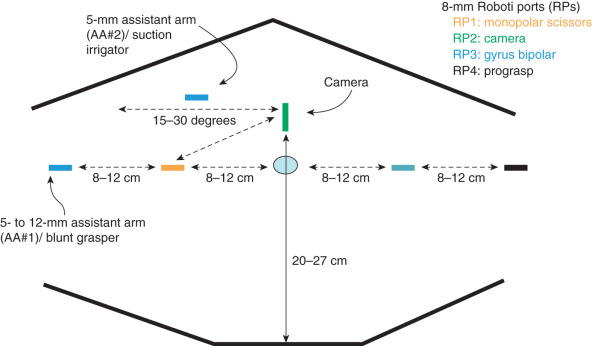
The port-site setup for robotic surgery is different from that for laparoscopic procedures because ports are generally placed above the umbilicus ( Fig. 21.3 ). Various port setups have been described for robotic gynecologic oncology surgery. Most gynecologic oncologists use one or two laparoscopic port and two additional laparoscopy ports to be controlled by the bedside assistant. In addition, the robot must be “docked” or attached to the ports, which is accomplished between the legs or from the side of the patient. When the surgical procedure commences, the experienced robotic surgeon can efficiently alternate (swap) control of the various robotic ports through a unique clutching system using both hand and foot controls, can operate all instruments in real time, and has direct control of both monopolar and bipolar electrosurgical energy sources. Although there is less reliance on the bedside assistant, that person is still instrumental in facilitating the case through robotic instrument changes, manipulation of vaginal instruments, suction irrigation, and use of an additional grasping instrument for retraction.
In addition to multiport surgery the options have expanded to single-port surgery or laparoendoscopic single-site surgery (LESS) and robotic single-site surgery. Single-port surgery uses a GelPort or Single Incision Laparoscopic Surgery (SILS) port in a 2- to 3-cm (umbilical) incision. Through this port, the camera port and usually two or three additional trocars are placed. Possible advantages include better cosmesis and decreased postoperative pain. Disadvantages such as port crowding, crossing of instruments, and need for advanced laparoscopic skills have limited the uptake for advanced and complicated pelvic surgeries. Robotic single site surgery allowed for computerized optical reversal of crossing instruments and hands with improved ergonomics and 3D visualization. Recently, an articulated needle driver has been added to the single-port platform, further improving surgeon comfort and easier applicability or single-site surgery. Cases have to be carefully selected because single ports will not allow for use in very obese patients with thick subcutaneous tissues, and the use of adequate uterine manipulators is imperative without the availability of ports to provide retraction and manipulation.
Surgical Procedure and Technique
After the ports are placed, actual surgical technique varies little except for the surgical steps required to complete various aspects of the procedure. After successful insufflation and placement of the trocars are accomplished, visual inspection of the abdominal cavity is undertaken, and the patient is placed in a steep Trendelenburg position. As in any surgical procedure, excellent exposure should be accomplished initially and maintained throughout the case. Use of steep Trendelenburg position is necessary, in lieu of packing the bowel, to achieve adequate visualization of the pelvis and lower abdominal region. Lysis of any adhesions holding the small bowel or omentum into the pelvis or lower abdomen should occur before beginning the pelvic and upper abdominal dissection. The small bowel is carefully placed in the upper abdomen by flipping the bowel from a caudad to a cranial position, exposing the mesentery of the small bowel and the aortic bifurcation ( Fig. 21.4 ). Care should be taken with this maneuver by using blunt instruments and gentle technique. In obese patients, body habitus may not allow a steep Trendelenburg position because of unacceptably high peak inspiratory pressure. Surgery may be able to be completed by decreasing the amount of Trendelenburg positioning (while the robot is undocked) or by decreasing the insufflation pressure slightly. Adjusting the respiratory frequency and tidal volume may further improve ventilation. In addition, obesity may prevent adequate mobilization of the small bowel out of the pelvis and upper abdomen to allow for retroperitoneal dissection. Some authors have advocated the use of additional port sites to help circumvent this problem; laparoscopic paddle or fan retractors may also improve exposure.
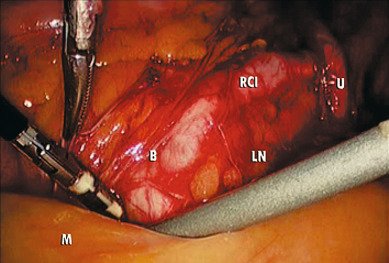
The key to successful advanced MIS is the same as that for open laparotomy: access to the retroperitoneum. In the pelvis, this is accomplished by dividing the round ligament laterally or opening the pelvic peritoneum lateral and parallel to the infundibulopelvic ligament. Some surgeons prefer to keep the round ligament intact so they can retract against it to keep the paravesical space open while dissecting tissue. Dissection is then carried down to the level of the external iliac artery, which is then followed in a cephalad and medial direction to the common iliac artery. At this point, the ureter can be found crossing the pelvic brim, and a window can be created between the ovarian vessels and the ureter ( Fig. 21.5 ). Development of the pararectal space is under direct visualization after identification of the bifurcation of the common iliac vessel and the ureter. The surgeon places traction on the ureter, medially developing the pararectal space between the hypogastric artery laterally and the ureter and rectum medially. Care must be taken during this dissection to avoid disrupting the cardinal web deep in the retroperitoneum. Because of the positive pressure environment of MIS resulting from the pneumoperitoneum, the boundaries of the paravesical space can be identified visually during laparoscopic surgery. The superior vesicle artery and umbilical artery are clearly visible as the medial umbilical ligament (see Fig. 21.2 ). Dissection is carried along the external iliac artery to the level of the superior vesicle artery. At this point, the superior vesicle artery is retracted in a medial direction, and the paravesical space is easily developed with the bladder and superior vesicle artery medially and external artery and obturator node bundle laterally. After this is accomplished, the uterine artery can be clearly identified at the origin of the superior vesicle artery from the hypogastric artery. This retroperitoneal pelvic dissection is the cornerstone of any laparoscopic surgery performed in the pelvis, including removal of an adnexal mass, LAVH, laparoscopic radical hysterectomy (LRH), and PLN dissection ( Fig. 21.6 ). Dissection can be facilitated with a variety of energy sources and clip appliers, each with its own advocates.
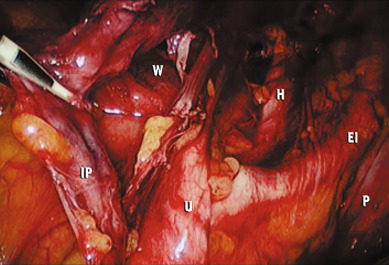

Extension of the incision along the peritoneum overlying the right common iliac artery and then along the aorta to the level of the duodenum allows for exposure of the paraaortic retroperitoneum ( Fig. 21.7 ). During this dissection, the peritoneum attached to the base of the cecum can be elevated in an anterior-cephalad direction, providing excellent exposure to the right paraaortic lymph node region. Margins of resection are identical to an open approach and can be extended to the level of the renal vessels. The left-sided paraaortic lymph node dissection requires dissection underneath the inferior mesenteric artery, mobilizing the descending colon and rectosigmoid off the left common iliac artery and retracting the inferior mesenteric artery and ureter laterally and cephalad. This gives excellent exposure to the left paraaortic lymph nodes inferior to the inferior mesenteric artery. Dissection can be continued above the inferior mesenteric artery in a similar manner. Some authors have advocated sacrificing the inferior mesenteric artery to get enhanced exposure to the upper left paraaortic nodes.

Unfortunately, the potential limitations previously described for MIS compared with laparotomy lead to the potential for inherent compromises in surgical technique. Therefore, the surgeon needs to obtain experience over time to optimize patient outcomes and emphasize excellent surgical technique. Knowledge of anatomy, the disease process, and surgical technique is key during these complicated surgical procedures. To minimize complications that are possible with any major surgical procedure, vigilance and meticulous surgical technique are required.
Applications of Minimally Invasive Surgery in Gynecologic Oncology
Cervical Cancer
The pioneering reports describing the use of advanced laparoscopic techniques in gynecologic oncology were initially described in patients with cervical cancer in which laparoscopy was used for PLN dissection in patients with early-stage disease to assess the feasibility for abdominal radical hysterectomy. One of the initial concerns about MIS in cervical cancer was that the laparoscopic approach would not be as thorough as laparotomy in assessing metastatic cancer and performing a comprehensive lymphadenectomy. Early studies in patients undergoing initial laparoscopic lymphadenectomy before laparotomy confirmed that a thorough pelvic and paraaortic lymphadenectomy is possible via LS, with no positive nodes discovered at laparotomy performed after laparoscopic lymphadenectomy. Nevertheless, the completeness of laparoscopic dissection was dependent on the experience of the surgeon, and the most common area for missed lymph nodes was lateral to the common iliac bifurcation. After the feasibility of laparoscopic lymphadenectomy was established, the use of MIS for radical surgery in patients with early-stage cervical cancer and for preradiation surgical assessment in patients with advanced disease was made possible.
Early-stage Cervical Cancer: Radical Hysterectomy
The establishment of the feasibility of laparoscopic lymphadenectomy led to the renewed interest in radical vaginal hysterectomy with laparoscopic lymphadenectomy and the development of novel minimally invasive radical hysterectomy techniques. Several groups worldwide have demonstrated the feasibility of the Schauta radical vaginal hysterectomy, laparoscopic-assisted radical vaginal hysterectomy (LAVRH), and total LRH with the goals of maintaining a high level of cancer control with improved patient outcomes in women with early-stage cervical cancer. The Schauta radical vaginal hysterectomy, described more than 100 years ago, and its subsequent modifications are performed completely vaginally; however, the surgeon does not have the opportunity to perform a retroperitoneal lymph node dissection. The LAVRH has many descriptions but usually involves a laparoscopic phase that includes the lymphadenectomy followed by developing the pararectal and paravesical spaces, dividing the parametria and paravaginal pedicles, and completing the remainder of the procedure vaginally. For the LRH, the entire radical pelvic operation is completed with MIS techniques. One advantage of the LAVRH over the LRH is the ability to precisely define the vaginal incision. Additionally, the performance of LAVRH allows for the development of the MIS skills required to perform a fertility-sparing radical vaginal trachelectomy. Currently, the favored laparoscopic approach is the LRH or robotic-assisted radical hysterectomy (RARH) because there is no need for a perineal phase for the performance of a vaginal incision, exposure is improved, visualization of critical anatomy and surgical planes is better, and anatomic limitations are fewer as a result of a narrow pelvis or lack of uterine descensus. Most important, however, is the fact that LRH mimics the well-established steps of an abdominal radical hysterectomy, thereby making LHR easier to adopt. Certainly, LRH is a technically challenging procedure. Most surgeons who use robotic techniques feel that the superior vision and wristed instruments provide optimal MIS capacity to perform the meticulous dissection required for the radical hysterectomy. Thus, the availability of the robotic platform allows for a significant shortening of the learning curve for MIS radical pelvic procedures and increased uptake of radical hysterectomy via the minimally invasive approach.
There is no doubt that the LARVH, LRH, and RARH are feasible for patients with early-stage cervical cancer. It has been demonstrated that these techniques can accomplish the oncologic goals of the surgery, allowing for the identification of critical anatomy, performance of a thorough lymph node dissection, and completion of the radical resection of the primary tumor with adequate margins. Most reports on LARVH and LRH demonstrate rates of complications comparable to those seen with the traditional open approach. A number of single-institution series reveal that the operative time is longer for LRH and RARH but associated with a decreased length of hospital stay ( Tables 21.1 and 21.2 ). More recent series are larger and also report recurrence and survival data, which compare favorably with matched historical patients undergoing a traditional radical hysterectomy for cervical cancer. A recent meta-analysis comparing abdominal radical hysterectomy with laparoscopic or robotic radical hysterectomy demonstrated less estimated blood loss, shorter length of stay (LOS), fewer wound-related complications, and febrile morbidity in patients undergoing minimally invasive radical hysterectomy. A large multi-institutional retrospective comparison reported similar findings with increased operating time for robotic radical hysterectomy compared with open radical hysterectomy but with lower blood loss and transfusion rates. Overall complication types were similar but occurred less frequently in the robotic radical hysterectomy group; intraoperative complications occurred in 4% versus 11%, and postoperative complications were seen in 30% versus 27% of patients undergoing robotic and open radical hysterectomy, respectively. Of note, recurrence and survival rates were similar. The traditional abdominal radical hysterectomy and the LRH/LARVH procedures are now being compared in a prospective randomized study, and recruitment is ongoing.
| Study | Patients (n) | Operating Room Time (h) | Blood Transfusion (%) | Intraoperative Complications (%) | Postoperative Complications (%) | Chronic Genitourinary (%) | Length of Stay (days) |
|---|---|---|---|---|---|---|---|
| Argentina, 1999 | 56 | 4.5 | NR | 2 | 6 | 2 | 4 |
| Quebec, 2000 | 54 | 4.5 | 4 | 7 | 10 | 0 | 5 |
| France, 2002 | 50 | 4.5 | 2 | 4 | 16 | 4 | 8 |
| France 2, 2002 | 95 | 4.0 | NR | NR | 12 | 2 | NR |
| WCC, 2002 | 78 | 3.5 | 1.3 | 9 | 9 | 1.3 | 3 |
| Germany, 2003 | 200 | 5.5 | 19 | 13 | 15 | 3.5 | NR |
| MSKCC, 2003 | 19 | 6.0 | 5 | 11 | 5 | 0 | 4.5 |
| Toronto, 2004 | 71 | 3.5 | 7 | 13 | 14 | 3 | 1 |
| MDA, 2007 | 35 | 5.75 | 11.0 | 11 | >20 | 0 | 2 |
| China, 2007 | 90 | 4.5 | NR | 9 | 40 | 3 | NR |
| Italy, 2009 | 65 | 3.25 | 0 | 5 | 10 | 1.5 | 4 |
| A. Laparoscopic | |||||||
| Reference (year) | Patients ( n ) | EBL (mL) | Operative Time (min) | LN Count | Intraoperative Complications ( n ) | Postoperative Complications ( n ) | Length of Stay (days) |
| 69 | 350 | 236 | 19 | 1 | n/a | 9 | |
| 42 | 145 | 216 | 20 | 1 | n/a | 10 | |
| 46 | 232 | 11 | 0 | 1 | 4.8 | ||
| 50 | 202 | 211 | 23 | 4 | n/a | 8.7 | |
| 76 | 95 | 255 | 38 | 2 | n/a | 4 | |
| B. Robotic | |||||||
| Reference (year) | Patients ( n ) | EBL (mL) | Operative Time (min) | LN Count | Intraoperative Complications ( n ) | Postoperative Complications ( n ) | Length of Stay (days) |
| 60 | 100 | n/a | 18 | 0 | n/a | 11 | |
| 73 | n/a | 152 | 11 | 0 | 3 | 4.1 | |
| 50 | 55 | n/a | 25 | 0 | n/a | 9.6 | |
| 63 | 50 | 213 | 29 | 1 | 2 | 1 | |
| 40 | 78 | 272 | 20 | 2 | n/a | 3.7 | |
| Boggess et al. (2008a,b) | 51 | 96.5 | 248 | 34 | n/a | 4 | 1 |
| C. Abdominal | |||||||
| Reference (year) | Patients ( n ) | EBL (mL) | Operative Time (min) | LN Count | Intraoperative Complications ( n ) | Postoperative Complications ( n ) | Length of Stay (days) |
| 176 | n/a | 168 | 16 | 1 | 9 | 9.6 | |
| 64 | 400 | 240 | 24 | 1 | 3 | 4 | |
| 40 | 222 | 200 | 26 | 5 | n/a | 5 | |
| Boggess (2008) | 49 | 417 | 211 | 23 | n/a | 8 | 3.2 |
Most authors stress that there is a lengthy learning curve for LRH and that complications decrease and overall operative efficiency improves as the surgeon gains experience. Therefore, these procedures should be performed by highly skilled and experienced surgeons in selected patients to obtain optimal outcomes. Of note, improvements in subspecialty training, collective and individual experience in performing the traditional abdominal radical hysterectomy, better perioperative care, and a general trend toward decreased hospital stay have demonstrated an overall improvement in the outcomes of patients undergoing the open abdominal approach. In fact, the shortest average length of hospital stay reported in many of the LRH series is similar to that reported in contemporaneous series of open radical hysterectomy. There is a lack of prospective data to compare cost and morbidity between the two approaches, and retrospective data do not definitively establish a significant advantage or disadvantage for the laparoscopic approach. Currently, it appears that with appropriate training and experience, minimally invasive radical hysterectomy is at least comparable to the open technique with regard to cost, morbidity, other perioperative outcomes, and survival.
Early-Stage Cervical Cancer: Fertility-Sparing Surgery
The ability to perform an adequate laparoscopic lymphadenectomy combined with the revival of the radical vaginal hysterectomy have led to the development of novel techniques for fertility preservation in young patients with early-stage cervical cancer. The laparoscopic vaginal radical trachelectomy (LVRT) is one of the most exciting applications of MIS in gynecologic oncology. In an effort to define the potential number of patients in whom this procedure would be considered, Sonoda and coworkers reported on 435 patients undergoing radical hysterectomy. Eighty-nine of these patients were younger than 40 years of age and had tumors that met the criteria for fertility-sparing radical trachelectomy (which represented 20% of their early-stage population) ( Table 21.3 ). This study clearly shows that there is a substantial population of patients who may benefit from this approach.
|
The LVRT technique combines laparoscopic pelvic and common iliac lymph node dissection with a radical vaginal trachelectomy (with preservation of the uterine fundus and creation of a neocervix). This procedure was initially described by ; since then, several centers have reported preliminary results on fertility-sparing radical trachelectomy with laparoscopic lymphadenectomy. Survival and fertility follow-up reports have been encouraging. To date, more than 300 cases have been reported with a recurrence rate of 4.1% and a death rate of 2.5%, which falls well within the range of survival seen with traditional radical hysterectomy in similar populations. Randomized prospective comparisons are not available, but case-control studies in matched patients reveal equivalent oncologic outcomes when comparing radical hysterectomy with radical trachelectomy. Plante et al. reported on the obstetric outcomes of 72 patients undergoing the surgery over a 12-year time span. A total of 50 pregnancies occurred in 31 women. Infertility rates and first and second trimester pregnancy loss did not appear to be increased. Of the patients reaching the third trimester, 22% had preterm delivery (although only 8% were delivered before 32 weeks). There appear to be enough data now published to consider fertility-sparing radical trachelectomy a viable option for select and motivated patients. Chuang and colleagues first reported robotic fertility-sparing radical trachelectomy in which the entire procedure is performed minimally invasively without a perineal or vaginal phase. Subsequent reports, limited by a small number of patients enrolled, have demonstrated the feasibility of robotic radical trachelectomy with decreased blood loss and LOS compared with open radical trachelectomy and without compromise of histopathologic or cancer outcomes. Approximately half of the patients who attempted pregnancy were able to conceive, and pregnancy rates were higher in the open surgery group. Whether this is due to shorter follow-up times in the MIS group or a true difference will have to be determined in future studies.
Advanced-Stage Cervical Cancer: Surgical Staging
Patients with advanced-stage disease or bulky early-stage cervical cancers are generally treated with definitive radiotherapy and concurrent chemotherapy. For this group of patients, laparoscopy has been used for surgical staging and to assist radiation oncologists in the safe placement of interstitial brachytherapy implants. Even positron emission tomography or computed tomography scans will not identify all patients with extrapelvic disease. Although controversial, surgical staging has been advocated to accurately define the extent of disease and guide the subsequent radiation fields. In patients with gross evidence of lymph node metastasis, their removal has been demonstrated in retrospective studies to improve survival compared with radiation of these nodes without debulking.
Before advanced MIS techniques, retroperitoneal lymphadenectomy for surgical staging of cervical cancer was performed at the time of extraperitoneal laparotomy. This approach afforded the surgeon excellent exposure to both the pelvic and paraaortic nodes with the ability to debulk grossly positive nodes. A transperitoneal laparotomy approach is not recommended because it is associated with a significant increase in the rate of severe postirradiation enteric morbidity compared with extraperitoneal laparotomy, presumably secondary to adhesion formation. Both transperitoneal and extraperitoneal laparoscopic approaches have been described for surgical staging of cervical cancer. Initially, there was concern that transperitoneal laparoscopic lymph node dissection would be associated with increased adhesion formation as experienced with transperitoneal laparotomy for lymphadenectomy. The extraperitoneal laparoscopic approach avoids this risk, but the surgeon only has access to the common iliac and paraaortic lymph nodes and is unable to perform a PLN dissection. However, transperitoneal laparoscopy, in general, is associated with fewer intraperitoneal adhesions compared with laparotomy. Blinded studies in animal models reveal a similar rate and severity of adhesions between transperitoneal laparoscopic lymph node dissection and extraperitoneal laparotomy; however, transperitoneal laparoscopic lymphadenectomy is associated with significantly fewer adhesions compared with transperitoneal laparotomy.
Multiple single-institutional series exist describing laparoscopy for pretreatment lymphadenectomy for advanced cervical cancer. The procedure is feasible with acceptable morbidity compared with laparotomy. A significant advantage for the laparoscopic approach is avoiding potential complications of a large abdominal incision and quicker postoperative recovery, allowing the patient to proceed to definitive radiation therapy more quickly. Although laparoscopic resection of nodes grossly involved with metastatic disease is technically feasible, it is definitely more difficult compared with laparotomy, especially when the nodes are fixed to vessels. Therefore, some surgeons prefer extraperitoneal laparotomy in case they encounter nodes that require debulking. The robotic platform obviates many of the inherent disadvantages of laparoscopy. As in laparoscopy, the robotic approach can be used for pelvic and paraaortic lymph node dissection. In addition, advantages of robotic surgery enable the surgeon to perform the more complicated debulking surgeries if necessary.
Endometrial Cancer
Laparoscopy
In the population of patients diagnosed with a gynecologic malignancy, MIS is most often applied for those diagnosed with endometrial cancer. Before the availability of advanced laparoscopic procedures, vaginal hysterectomy was a less invasive option for definitive surgical therapy, especially in patients with severe comorbidities and an increased risk of complications secondary to laparotomy. In fact, assuming the disease is confined to the uterus, the curative potential of the hysterectomy should be equivalent regardless of the surgical approach. The disadvantages of the vaginal hysterectomy in endometrial cancer include the inability to fully inspect the peritoneal cavity and retroperitoneum (lymph nodes) for metastatic disease and potential inability to complete BSO. The addition of laparoscopy to the vaginal hysterectomy essentially eliminates these disadvantages. Most patients with endometrial cancer present with apparent early-stage disease. Approximately 15% to 25% will be upstaged as a result of surgical staging. The majority of patients with extrauterine disease have occult (without gross evidence) spread. Current disease assessment modalities such as preoperative imaging, intraoperative palpation, gross inspection, and frozen section of the uterus all are inaccurate compared with comprehensive surgical staging that includes bilateral pelvic and paraaortic lymph node dissection. Comprehensive surgical staging with lymphadenectomy provides the best definition of the biologic nature of the disease and allows the oncologist to make informed postoperative treatment decisions. Laparoscopic-assisted vaginal hysterectomy bilateral salpingo-oophorectomy (LAVH-BSO) or total laparoscopic hysterectomy (TLH) can be substituted for total abdominal hysterectomy bilateral salpingo-oophorectomy (TAH-BSO) in the algorithm presented earlier in this book for the management of endometrial cancer.
The overall management of patients with apparent early-stage endometrial cancer continues to evolve. Endometrial cancer remains the least uniformly managed gynecologic malignancy, even among gynecologic oncologists. It remains controversial whether all patients should have surgical lymph node assessment and, in those undergoing lymphadenectomy, the extent to which lymph node dissection should be performed. Nevertheless, until laparoscopic lymph node dissection was described, comprehensive surgical staging of endometrial cancer by MIS was not feasible. The earliest series published on MIS management of endometrial cancer primarily described pelvic lymphadenectomy with a minimum of patients undergoing a paraaortic lymph node dissection. The ability to perform laparoscopic lymphadenectomy evolved from pelvic nodes to right-sided paraaortic nodes to a bilateral paraaortic lymph node dissection to the level of the inferior mesenteric artery. Although controversial, recent reports on surgical staging of endometrial cancer recommend that selected patients at high risk for extrauterine disease have a bilateral paraaortic dissection to the level of the renal vessels. This technique has been described laparoscopically but is technically difficult and likely currently applied to a small minority of patients undergoing MIS management of endometrial cancer.
Until recently, most data describing the experience of laparoscopy in management of endometrial cancer came from single institutions and are primarily retrospective studies. It is clear that advances in MIS technology, training, and experience allow the surgeon the opportunity to ensure removal of the uterus and adnexae, inspect the peritoneal cavity, define the retroperitoneal anatomy, and perform a thorough pelvic and paraaortic lymph node dissection. The reported benefits of a laparoscopic approach in endometrial cancer are lower blood loss and transfusion rates, shorter hospital stay, faster postoperative recovery, and superior short-term QoL, albeit at the expense of longer operative times. However, these studies are biased by the fact that they represent only selected patients (eg, those with low body mass indexes [BMIs]) compared with an institution’s entire population of patients with endometrial cancer. Additionally, these series vary as to whether comprehensive surgical staging was performed and, if so, as to the extent of lymph node dissection ( Table 21.4 ). Surgeon experience and patient factors such as obesity or previous abdominal surgery contribute to the many disadvantages of the laparoscopic approach in endometrial cancer, especially if the goal is comprehensive surgical staging. In a study of two California databases from 1997 to 2001, only 7.7% of patients diagnosed with endometrial cancer underwent surgery via a laparoscopic approach. A recent survey of members of the Society of Gynecologic Oncologists (29% responding) revealed that use of MIS as a surgical treatment option for endometrial cancer has continued to increase, with almost 70% of gynecologic oncologists stating that this is the procedure they perform most via the MIS approach.
| A. Robotic | ||||||||
| Reference (year) | Patients ( n ) | EBL (mL) | BMI (kg/m 2 ) | Conversion to Laparotomy (%) | LN Count | Intraoperative Complications (%) | Postoperative Complications (%) | Length of Stay (days) |
| 377 | 47 | n/a | 3.5 | 15.5 | 0.5 | 5.9 | 1.4 | |
| 503 | 90 | 32 | 6.4 | 1.6 | 7.6 | 1 | ||
| Boggess et al. (2008a,b) | 103 | 75 | 33 | 3 | 33 | 1 | 5 | 1 |
| 102 | 109 | n/a | 1 | n/a | 2 | 8.8 | 1.9 | |
| Lowe et al. (2009a,b) | 405 | 88 | 32 | 6.7 | 15.5 | 3.5 | 14.6 | 1.8 |
| Seamon et al. (2009a,b,c,d) | 105 | 100 | 34 | 12 | 31 | n/a | n/a | 1 |
| Seamon et al. (2009a,b,c,d) | 109 | 109 | 40 | 15. | 25 | 2.2 | 11 | 1 |
| B. Abdominal | |||||||
| Reference (year) | Patients ( n ) | EBL (mL) | BMI (kg/m 2 ) | LN Count | Intraoperative Complications (%) | Postoperative Complications (%) | Length of Stay (days) |
| 131 | 198 | NR | 12.1 | 3 | 21 | 5.3 | |
| Boggess et al. (2008a,b) | 138 | 266 | 35 | 14.9 | 0.7 | 29 | 4.4 |
| 131 | 13 | 32 | 13.1 | n/a | 20.6 | 5 | |
| Seamon et al. (2009a,b,c,d) | 191 | 394 | 40 | 24 | 2.5 | 27 | 3 |
| C. Laparoscopic | ||||||||
| Reference (year) | Patients ( n ) | EBL (mL) | BMI (kg/m 2 ) | Conversion to Laparotomy | LN Count | Intraoperative Complications (%) | Postoperative Complications (%) | Length of Stay (days) |
| Boggess et al. (2008a,b) | 81 | 146 | 29 | 5% | 23.1 | 3.7 | 10 | 1.2 |
| 173 | 187 | n/a | 5.2% | n/a | 3.5 | 7.5 | 2.3 | |
| Seamon et al. (2009a,b,c,d) | 76 | 250 | 29 | 26% | 33 | 2.6 | n/a | 2 |
Several prospective randomized trials comparing laparoscopic and open surgery for endometrial cancer staging have been conducted. In 2009, the Gynecologic Oncology Group (GOG) reported the results of the LAP-2 (Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study) trial, which enrolled 2531 patients with apparent early-stage endometrial cancer. All patients in this study were to undergo complete surgical staging and were randomly assigned in a 2 : 1 ratio of laparoscopy to laparotomy. Of the 1678 patients on the laparoscopy arm, 25.8% required conversion to laparotomy (of note, conversion to laparotomy was mandatory if lymph node dissection could not be completed laparoscopically). More than half of these conversions were a result of poor visualization (exposure), 16% were from metastasis, and 11% were from bleeding. Fig. 21.8 graphs the likelihood of converting from laparoscopy to laparotomy as a function of BMI, age, and evidence of metastatic disease. In this landmark study, there was no significant difference in rate of node positivity between the two groups; however, significantly fewer patients in the laparoscopy arm (78.5%) compared with the laparotomy arm (86.4%) had lymph nodes histologically identified from all four primary nodal regions (which include the right and left periaortic and bilateral PLNs). The incidence of intraoperative complications, reoperations, and readmissions was similar, but significantly fewer Common Toxicity Criteria grade II or greater postoperative complications ( P <0.0001) occurred on the laparoscopy arm (14.3%) compared with the laparotomy arm (21.1%). Similar transfusion rates were seen between the groups (7% to 9%). Consistent with other studies in the literature, operative time was significantly longer for the laparoscopic arm (203 minutes) versus the laparotomy arm (136 minutes). For patients successfully completing laparoscopic surgery, the average length of hospital stay was 2 days versus 4 days for laparotomy ( P <0.0001). During the perioperative period, the LAP-2 study revealed significantly better overall QoL, pain scores, resumption of normal activities, and time until return to work in the laparoscopy arm. By 6 months after surgery, no significant QoL differences were found except in that of body image. Previously reported but small single-institution studies reveal no differences in survival between laparoscopy and laparotomy for the surgical management of endometrial cancer. LAP-2 also examined this most important survival endpoint. The estimated 3-year overall survival rates from this study are 89.8% for laparoscopy and 89.9% for laparotomy, with similar rates of recurrence and death from disease. Other international studies such as the Dutch study by Mourits et al. in which patients were randomized to TLH versus abdominal hysterectomy (without lymph node staging) and the Australian study (Laparoscopic Approach to Cancer of the Endometrium [LACE] trial) by Janda and coworkers, which also included pelvic lymphadenectomy reported similar findings. The latter also found better QoL up to 6 months after TLH and with fewer complications.
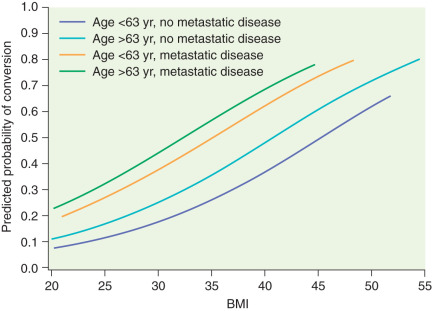
Laparoscopy offers selected patients with endometrial cancer the potential benefits of shorter hospital stay, less need for blood transfusion, and decreased postoperative complications with a similar risk of intraoperative complications. Nevertheless, laparoscopic hysterectomy with pelvic and paraaortic lymph node dissection remains difficult to learn and is least likely to be offered or successful in patients with obesity, the group of patients most likely to benefit from MIS. Although morbid obesity is the most common risk factor for developing endometrial cancer, it is also the major limitation to successful laparoscopic surgical management. Most studies reporting the use of MIS for the treatment of endometrial cancer describe a population of patients with a mean BMI lower than that typically seen in the overall population of patients with endometrial cancer. Even the LAP-2 study represents a selected group of patients because the median BMI was 28 kg/m 2 in both arms. The rate of conversion from laparoscopy to laparotomy ranges quite widely between single-institution reports. Variables such as intent to perform comprehensive staging, adhesions, intraoperative complications, discovery of metastatic disease, and surgeon experience have all been reported as important factors. In the LAP-2 study, the overall conversion rate was 25.8% and strongly influenced by BMI (with a rate of 17.5% in patients with a BMI of 25 kg/m 2 and a rate of 57% in patients with a BMI greater than 40 kg/m 2 ).
Robotics
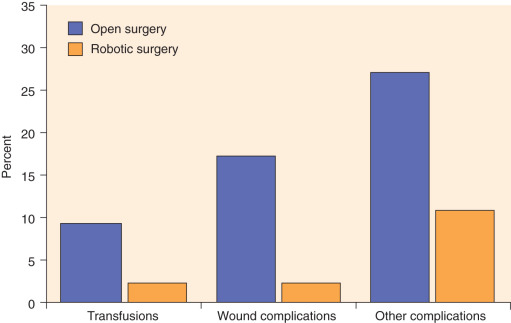
As previously mentioned, recent developments in robotic technology have allowed the surgeon to gain advantages compared with laparoscopy, and this technology has rapidly been adopted by gynecologic oncologists independent of their prior laparoscopic experience. Relatively large single-institution and pooled reports reveal that the robotic surgical staging of patients with endometrial cancer is feasible and that the patient experiences the typical benefits of MIS compared with laparotomy. Furthermore, these reports have shown that robotic surgery is likely applicable to a larger portion of patients diagnosed with endometrial cancer compared with laparoscopy, including patients who are morbidly obese (see Table 21.4 ). Seamon and colleagues demonstrated that comprehensive surgical staging is feasible in a relatively heavy group of patients (mean BMI, 34 kg/m 2 ), as robotic pelvic and paraaortic dissection was performed in 85% of the patients overall and in 67% of the patients with BMI more than 45 kg/m 2 . Gehrig and coworkers compared their robotic and laparoscopic experience in obese patients (BMI >30 kg/m 2 ) with endometrial cancer and demonstrated a significant difference in hospital stay, blood loss, and operating room time in favor of the robotic group. Seamon and colleagues compared 105 patients with endometrial cancer undergoing robotic surgery with 76 patients managed laparoscopically and revealed a similar ability to complete surgical staging. However, comprehensive surgical staging was able to be performed in heavier patients compared with laparoscopy (BMI, 34 kg/m 2 vs. 29 kg/m 2 ), and with significantly shorter LOS, lower conversion to laparotomy, and shorter operating room time in favor of the robotic group. Compared with laparotomy, the advantages of the robotic approach are more dramatic in obese patients with endometrial cancer. In a matched cohort study in patients with a median BMI of 40 kg/m 2 and multiple comorbidities, patients managed robotically experienced a decreased LOS (1 vs. 3 days) and significantly lower rates of overall complications (odds ratio [OR], 0.29), blood transfusion (OR, 0.22), and wound complications (OR, 0.10) compared with laparoscopy ( Fig. 21.9 ). Even in extreme morbid obese patients (mean BMIs, 48, 51, and 54 for laparoscopic, robotic, and open hysterectomy, respectively), MIS (laparoscopic and robotic) was associated with lower rates of blood loss and shorter hospital stays despite longer operative times. Similarly, comorbidities, not weight and BMI, were predictive of an increased risk of perioperative complications.
In addition, recurrence rates and survival appear similar to open surgery in retrospective reviews. Although it is most critical that the surgeon have expertise in the management of patients with endometrial cancer, it appears that the learning curve for comprehensive robotic staging of endometrial cancer is not as difficult as laparoscopy. Experiences from several institutions have shown that 10 to 20 cases are needed to gain proficiency with the procedure. Operative times improve as the surgeon and the surgical team become more familiar with the setup and techniques. The robotic platform is now the dominant surgical approach in the management of women with apparent early-stage uterine cancer.
Single-Site Surgery
In 2009, Fader and Escobar reported their initial experience with LESS for gynecologic cancer surgery. Larger series have since been published and have demonstrated safety and feasibility of the single-site approach for endometrial cancer. Although single-site surgery has been reported in moderately obese patients (median BMI, 32–33), abdominal wall thickness remains a limiting factor for the single port in extreme morbidly obese patients.
Minimally Invasive Sentinel Lymph Node Assessment
In an attempt to reduce the risk of side effects and complications (bleeding, lymphocele, lymphedema, longer operating time) seen with comprehensive lymph node dissection, sentinel lymph node (SLN) assessment has been developed and has been part of standard of care in breast cancer, melanoma and vulvar cancer for several years. SLN assessment is now also an acceptable part of standard of care in women with endometrial cancer and cervical cancer and has been incorporated in the National Comprehensive Cancer Network’s guidelines (version 2.2015). SLN assessment can be performed using open and minimally invasive techniques, and equipment is available for laparotomy, laparoscopic, and robotic approaches. Blue dye (isosulfan blue, patent blue, methylene blue) has been studied most extensively. Other available methods include radiolabeled technetium-99 with lymphoscintigraphy, indocyanine green dye (ICG) with immunofluorescence detection, and a combination of the various methods. Some studies advocate for using ICG only given the high detection rates and ease of administration as well as a low risk of allergic reaction (1:40,000). For cervical cancer, peritumoral injection is intuitive and relatively easy. In contrast, the best location of injection is not as clear for endometrial cancer. Investigators have studied hysteroscopic tumor injection, fundal or subserosal injection, cervical injection, or a combination of multiple injection sites. Although combined location injection methods have improved SLN detection rates, the overall accuracy of intracervical injection alone is high. Certainly, some surgeons have expressed concern about missing aortic SLNs, but staging studies have demonstrated that the risk of isolated positive aortic lymph nodes in the setting of negative PLNs is rare (1%–2%). However, it is important to keep in mind that in high-risk patients (high-grade histology or deep myometrial invasion), the risk of paraaortic lymph node involvement increases to 4%, and as such, the safety of intracervical injection for SLN assessment in high-risk patients has not yet been confirmed. Most surgeons prefer to perform cervical injections only, and injection protocols vary. In general, 1 mL of dye is injected at the 3 and 9 o’clock position. Some believe dye should be injected superficially (submucosal) and deep (intrastromal), but others inject superficially only. Next, ports are placed, and the retroperitoneal spaces are developed. Care should be taken to maintain excellent hemostasis because blood can obscure visualization of the various types of dye. SLNs are dissected, labeled for location, and sent separately. Detection rates (any detection) vary from 80% to 95% in endometrial cancer and 84% to 99% in cervical cancer depending on the study and methods used, and best detection in cervical cancer is seen in tumors smaller than 2 cm. Bilateral detection varies between 60% and 85% for cervical cancer and 52% and 79% in endometrial cancer. Adherence to an SLN algorithm is critical for successful application of SLN assessment; all suspicious or enlarged lymph nodes should be removed, and a site-specific complete lymphadenectomy is performed when SLNs are not identified. When adhering to these principles, the negative predictive value is high (95%–100%), and false-negative rates are low. Most institutions use ultrastaging (serial sectioning of the SLNs with immunohistochemistry in addition to standard hematoxylin and eosin stain) to assess for metastatic disease; however, the clinical significance of micrometastases detected with ultrastaging has not been defined at this time.
Removal of a Large Uterus
If the uterus is too large or the vaginal opening is too small to remove the uterus through the vagina, the surgeon can place the uterus in a laparoscopic bag (through the vagina or a port site). Sometimes this allows for removal of the uterus through the vagina, providing a smoother outer surface and more equal distribution of pulling forces. If still unsuccessful, the midline camera port can be slightly extended to allow for uterine removal in the laparoscopic bag (mini-laparotomy). Some surgeons bivalve or morcellate the uterus in the bag; however, removing the uterus intact for complete pathologic assessment is preferred. Certainly, power morcellation is not recommended when operating on patients with invasive or preinvasive disease.
Uterine Manipulation for Minimally Invasive Surgery
Adequate uterine and vaginal manipulation is a requirement for successful completion of MIS to identify the correct planes of dissection and to avoid additional ports to retract and move the uterus. Early studies reported increased incidence of positive peritoneal cytology after laparoscopic hysterectomy (10%) compared with open hysterectomy (2.8%). However, more recently, two prospective studies comparing pelvic washings before and after insertion of the uterine manipulator found no significant increase (0%–4%) in positive cytology after insertion of the manipulator. Tubal contamination was reported to be higher after robotic compared with laparoscopic hysterectomy, and avoiding manipulation of the tubes but rather retracting using the round ligaments may be considered. There has been no evidence that true lymphovascular invasion is more common with the use of uterine manipulators in endometrial or cervical cancer. Most important, there is no evidence that the use of a uterine manipulator leads to increased risk of recurrence or worse prognosis, although randomized studies have not been performed.
Adnexal Mass
One of the most common clinical scenarios presented to gynecologic surgeons is that of the suspicious adnexal mass. Because most masses are asymptomatic, the major reason for surgical removal is to determine if a malignancy is present. The finding of a persistent ovarian mass represents a major reason for surgery in gynecology. Killackey and Neuwirth reported that 17% of laparotomies in gynecology are performed primarily for this indication. It has been reported that 5% of persistent premenopausal ovarian masses and 20% to 50% of those in postmenopausal women are malignant. This risk of malignancy (and potential need for surgical staging or cytoreduction) has led to the recommendation that suspicious ovarian masses be removed through a vertical midline incision. This recommendation, however, is made with the assumption that a gynecologic oncologist or other trained surgeon will be available to provide surgical support if required. This surgery may include radical pelvic or upper abdominal dissection, retroperitoneal lymphadenectomy or lymph node debulking, or bowel resection. Most gynecologists do not have such assistance on standby for every surgery performed for a pelvic mass. Potential advantages of minimally invasive approaches to the pelvic masses are cost savings and decreased morbidity in those women without cancer and early diagnosis and referral for appropriate surgical management in those who are found to have malignancies.
Postmenopausal women undergoing laparotomy for ovarian masses have a much greater likelihood of malignancy than premenopausal women. Postmenopausal patients with ovarian masses differ from premenopausal patients with respect to the predictive value of CA-125. An elevated CA-125 level has a positive predictive value for malignancy of 80% to 98% in this population. Postmenopausal patients with an adnexal mass and an elevated CA-125 level are presumed to have a malignancy and should have surgery by an ovarian cancer specialist regardless of the ultrasound findings. The ability to identify patients preoperatively at highest risk for cancer (with the appropriate selection of surgical approach and consultation or referral for surgical management of this potential cancer) is often challenging. Although pelvic ultrasonography, tumor markers, and clinical presentation all contribute to risk stratification for ovarian cancer, the definitive evaluation of a mass is determined during surgery. However, multiple scoring systems have been devised to provide the surgeon with a preoperative probability of a mass being malignant. The SGO and the American College of Obstetrics and Gynecology jointly published referral guidelines and management recommendations for patients who present with a pelvic mass. Chapter 10 includes a comprehensive discussion of risk assessment of an adnexal mass in both premenopausal and postmenopausal women. In general, if a surgeon stratifies the risk of an adnexal mass into low-, medium-, and high-risk categories for malignancy, then the risk of finding an “unexpected” ovarian cancer at the time of surgery should be extremely low.
Minimally Invasive Surgery Management
Several authors have reported on the LS management of suspected benign ovarian masses. In 1992, Nezhat et al. reported 1209 adnexal masses managed laparoscopically. The majority of patients had endometriosis or functional cysts. However, 64 patients had benign ovarian tumors, and four were malignant. There were no reported major complications in removing masses up to 25 cm in diameter, clearly demonstrating the technical feasibility of managing ovarian masses laparoscopically. Since then, multiple authors have reported on the use of laparoscopic oophorectomy in both premenopausal and postmenopausal women. The complication rates in these nonrandomized reviews of laparoscopic surgery vary from 0% to 18% and include bowel injury, ureteral injury, wound infection, hematoma, and hemorrhage. A consensus of these retrospective reviews and small randomized trials is that laparoscopic management of adnexal masses is associated with decreased or similar operating time and decreased perioperative morbidity, including pain, infection, and blood loss compared with laparotomy. These studies also show a decreased LOS and potential cost savings; however, major complications are still possible.
More recently, authors have reported on using laparoscopy in the initial management of ovarian masses suspicious for malignancy. Dottino and coworkers reported on 160 patients with suspicious adnexal masses who had no evidence of gross metastases or extension above the umbilicus. No distinction was made based on other risk factors for malignancy; however, all of these patients were referred for gynecologic oncology consultation. A total of 141 patients were successfully managed laparoscopically. Invasive ovarian cancer was discovered in nine patients, borderline ovarian tumors in eight, and nongynecologic cancer in four. Dottino and associates reported a 3% incidence of intraoperative complications requiring conversion to laparotomy and only one incidence of intraoperative spillage of tumor. This was a sex cord–stromal tumor, which did recur locally. Canis et al. reported on 230 adnexal masses suspicious or solid at ultrasound examination evaluated initially by laparoscopy. Twenty percent of the invasive cancers and 50% of the borderline tumors had cyst puncture or rupture at the time of diagnosis. One case of tumor dissemination occurred with morcellation of an immature teratoma. These studies highlight the need to prevent tumor spill and morcellation for all suspicious masses. There is concern that the positive-pressure carbon dioxide environment established during pneumoperitoneum may predispose patients to intraperitoneal seeding. Animal studies have shown an increased seeding rate in the pneumoperitoneum group compared with control participants. This may be explained by peritoneal damage and exposure of the underlying basal lamina, which could facilitate implantation. No clear conclusions can be drawn regarding the risk to humans, but these studies suggest cyst rupture or spillage should be avoided in all ovarian masses that could possibly be malignant.
The algorithm presented in Fig. 21.10 is based on a strategy of maximal use of MIS combined with minimal risk of unexpected finding of ovarian cancer or intraabdominal tumor spill. The presence of a unilocular cystic mass in premenopausal women is rarely malignant regardless of size. This fact is important because most of these patients are candidates for ovarian conservation with cystectomy. The same criteria for conservative management can be used with MIS as is already being done with laparotomy. Cystectomy has been described using a variety of techniques using MIS. Equipment such as needle aspirators and intraabdominal bagging devices can allow for cyst decompression without spillage. Techniques have been described for either transabdominal or transvaginal cyst aspiration and removal. Large cysts can be aspirated transvaginally and removed via a posterior colpotomy with no intraabdominal spillage. Size limits for removal of unilocular cysts seem to be related to safety of trocar insertion, surgical exposure, and experience of the surgeon. If oophorectomy is to be performed, the aspiration of the unilocular cyst will allow most of these to be removed through the anterior abdominal wall trocar site. More complex cysts that are believed to be benign can be removed in a bag through a colpotomy or by extending the midline port-site incision at the end of the case. Some multilocular ovarian neoplasms larger than 8 cm in size that are thought to be benign based on ultrasonography have a large dominant cyst. These can be managed through a posterior colpotomy with transvaginal drainage of the dominant cyst and subsequent removal of the ovary through the colpotomy. Suspicious masses should not be intentionally aspirated or morcellated outside of a controlled situation that prevents tumor spill and intraperitoneal dissemination.
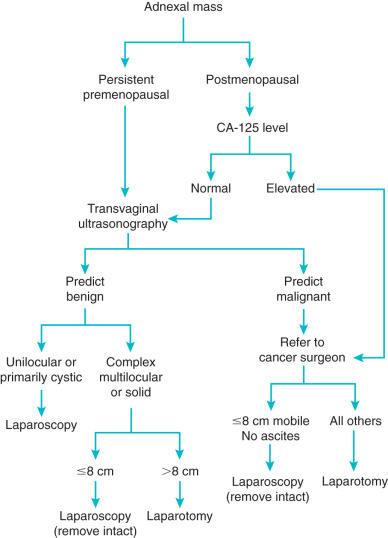
If known ultrasound criteria are used, the negative predictive value for ovarian malignancy in premenopausal women should be greater than 95%. If the schema is adhered to, the few women with malignancies should have their tumors removed with no intraabdominal spillage (see Fig. 21.10 ). The rare finding of malignancy will still allow for appropriate timely referral, surgical staging, and treatment. Because the majority of premenopausal women have masses with benign characteristics, this approach could substantially reduce the number of laparotomies performed for ovarian masses.
When the transvaginal ultrasound examination is consistent with probable malignancy, a different approach is recommended (see Fig. 21.10 ). The positive predictive value of ultrasonogarphy alone ranges from 30% to 80%. Patients should have primary therapy by a physician trained to deal with ovarian cancer surgery and staging. These tumors can be managed with MIS if they are 8 cm or smaller and can be removed intact (and in a bag) through a colpotomy or mini-laparotomy. If malignancy is documented, these patients should have further surgery by appropriately trained physicians as soon as possible. Patients with ovarian masses larger than 8 cm that are suspicious for malignancy or those that cannot be removed without controlled rupture by laparoscopy should undergo laparotomy by ovarian cancer specialists. Patients who are found to have disease spread beyond the ovary at time of MIS should have a confirmatory biopsy of the extraovarian disease (higher yield than biopsy of a cyst) and referral for definitive therapy and staging as soon as possible.
Ovarian Cancer
Diagnosis of Ovarian Cancer
As a result of the relatively poor ability to predict malignancy with existing preoperative technology, a number of studies have looked at using laparoscopy to assess the presence or absence of ovarian cancer. Aspiration cytology of ovarian cyst fluid has a poor negative predictive value in the range of 58% to 80%. Case reports have raised the possibility that aspiration or biopsy of malignant ovarian cysts may lead to peritoneal tumor implantation, limiting its application for diagnostic purposes. Also, use of therapeutic aspiration is ineffective for resolution of an ovarian cyst, with a recurrence rate as high as 67%. Therefore, routine or intentional cyst aspiration is not recommended, especially in the presence of a suspicious mass. Reports of success are likely to include functional cysts because aspiration does nothing to interrupt the pathologic process of an ovarian neoplasm or endometrioma. When laparoscopic biopsy is highly suspicious for malignancy, definitive surgery should be performed if a trained ovarian cancer surgeon is available; otherwise, the surgery should be aborted, and the patient should be referred immediately to such a specialist.
Ovarian Cyst Rupture
The main concern about managing a suspicious adnexal mass with MIS, especially when a cancer is apparently confined to the ovary, is that rupture may cause dissemination of malignant cells into the peritoneal cavity and adversely affect surgical stage and thus survival. MIS is more likely than laparotomy to result in capsular rupture because it potentially includes more tumor manipulation, and masses often must be drained before removal from the peritoneal cavity. In an assessment of outcomes in the laparoscopic management for adnexal masses, both benign and malignant, mass rupture occurred in 25% of the cases. Therefore, use of laparoscopy has been discouraged because of the potential for rupture of a malignant ovarian cyst. However, the prognostic impact of cyst rupture in an encapsulated stage I ovarian cancer remains controversial. Any spillage should be managed with copious irrigation, as is the case with open surgery.
Another concern regarding MIS for ovarian cancer has been the possible effects of a delay in referral for subsequent definitive surgery and staging after an initial diagnosis of cancer. In an early report by Maiman et al. of 42 patients referred for malignant ovarian neoplasms excised laparoscopically, the mean interval to laparotomy was 5 weeks, and more than 10% of these patients did not undergo subsequent exploratory surgery. The impact of this delay on survival is not known. In a review of 48 cases of surgical staging after laparoscopic removal of malignant ovarian masses, Lehner and associates reported that a delay of more than 17 days was associated with an increased risk of advanced-stage disease for malignant and low-malignant-potential tumors on univariate analysis. These concerns are heightened by Kindermann et al.’s retrospective survey of 192 cases of ovarian malignancy initially diagnosed laparoscopically. Patients with a delay of more than 8 days between laparoscopic biopsy and definitive surgery had an increased risk of port-site metastasis and progression to stage III disease. In this report, only 7% of the apparent stage I tumors were removed intact as a result of biopsy, capsule rupture, and morcellation, making interpretation of these data difficult. These findings suggest that all efforts should be made to avoid intraoperative spillage from the ovary and to limit delay until definitive surgery. If patients are managed according to Fig. 21.10 , the unexpected finding of ovarian malignancy should be less than 5%. The few malignant masses that are managed with MIS should be removed without intraabdominal rupture, and survival is unlikely to be negatively affected. Managing patients according to this schema allows for appropriate and timely referral of patients with ovarian malignancy to physicians appropriately trained to manage these patients.
Early-stage Ovarian Cancer
The location and frequency of subclinical metastasis in patients with presumed early-stage ovarian cancer have encouraged investigation of the use of laparoscopic surgery for this patient population. Many areas traditionally evaluated during open laparotomy can be adequately assessed via MIS, including peritoneal cytology, the diaphragm, the omentum, the pelvic and paraaortic lymph nodes, and the pelvic peritoneum. Areas that are less likely to be fully visualized laparoscopically include the abdominal peritoneum and the bowel mesentery and serosa secondary to less ability to “run the bowel” and the inability to palpate. Review of the literature indicates that the most common sites of subclinical metastasis in ovarian cancer include peritoneal cytology, the pelvic and paraaortic lymph nodes, the diaphragm, and the pelvic peritoneum, all of which can be adequately evaluated laparoscopically or robotically (see Table 11.16 ). The inability to thoroughly evaluate the abdominal peritoneum and bowel mesentery could potentially lead to a 3% to 5% risk of understaging. Laparoscopic staging may be particularly helpful in patients who have undergone a recent exploratory laparotomy for removal of an adnexal mass found to be malignant who did not have appropriate staging. In 1995, Childers et al. reported on the technical feasibility of laparoscopic staging of presumed stage I ovarian cancer. They found metastatic disease in eight of 14 patients, including three with positive paraaortic nodes, three with pelvic disease, and two with positive cytology. Following this report, several small series of laparoscopic staging in this patient population have been published. In a case-controlled series, Chi and coworkers demonstrated equivalent node counts and omental size removed in patients undergoing laparoscopic versus open procedures. There was no significant difference in the rate of metastatic disease between the groups, although the numbers are too small to provide adequate power.
To date, there are no randomized trials looking at surgical staging or survival outcomes in early-stage ovarian cancer managed laparoscopically. An attempted Cochrane meta-analysis in 2009 was unsuccessful because of the low number of studies in the literature. In 2013, Park and associates conducted a meta-analysis of laparoscopic staging in early ovarian cancer and were able to include 11 observational studies. Operative outcomes were similar to those of laparotomy. The overall rates of upstaging and conversion to laparotomy were 22.6% and 3%, respectively, and the tumor rupture was reported at 25.4%. One port-site metastasis was found. A large multi-institutional retrospective review included 300 patients with apparent early-stage ovarian cancer. It compared 150 patients who underwent laparoscopic staging at initial diagnosis with 150 patients who had delayed staging after incidental diagnosis. Patients in the delayed staging group were younger and more likely to be premenopausal with a possible desire for fertility-sparing surgery. Conversion to laparotomy and tumor spillage was higher in the group with immediate staging (16% and 12% vs. 2% and 2%) and upstaging was three times more common (24.9% vs. 7.4%) in the immediate staging group, which also included more poorly differentiated tumors. There were no differences in complication rates, disease-free survival, or recurrence, although the follow-up time was short. No port-site recurrences were identified. These studies and other small studies demonstrate that laparoscopic staging is feasible and safe for apparent early-stage ovarian cancer. Peritoneal surface metastases are the most challenging to identify with limited ability to manipulate tissues during laparoscopic surgery.
Advanced Ovarian Cancer
Minimally invasive surgery has had a limited role in the management of advanced ovarian cancer. However, more recently, there has been a renewed interest in incorporating MIS as part of the initial evaluation of patients with suspected advanced ovarian cancer. Since the early 1980s, authors have reported its role in second-look laparotomy. Initial studies from the early 1980s using laparoscopy followed by immediate laparotomy indicated a false-negative predictive value of 29% to 55% for laparoscopic surgery. With advances in video equipment and surgical technology, there was a brief resurgence of use of laparoscopic surgery in the second look setting. Even using more modern minimally invasive technology, the negative predictive value was 86%, indicating continued deficiencies in the technique. In addition, complete intraperitoneal investigation was only successful in 41% of patients because of adhesions from prior surgery. This information, in combination with the fact that second-look surgery has been determined to be of no therapeutic value, caused laparoscopy for second look to fall out of favor.
Several studies have emphasized the importance of a complete cytoreduction to no gross residual disease for optimal cancer outcomes and survival. However, if optimal or complete cytoreduction is not feasible because of disease distribution or patient factors, neoadjuvant chemotherapy can be considered. To determine the extent of disease in and the feasibility of primary cytoreductive surgery, laparoscopy can be useful. Several algorithms have been developed, some including just findings at laparoscopy and some using radiologic and serologic markers. Using laparoscopy, exact tumor location, extent of tumor spread, and invasion in surrounding structures are assessed, and each parameter receives a score. Findings at laparoscopy have been demonstrated to correlate well with findings at laparotomy, and the total score provides the surgeon with an estimated chance of successful primary debulking surgery to no gross residual disease. Prospective trials are currently ongoing to evaluate the safety, feasibility, and predictive values of laparoscopy to determine resectability at the time of ovarian cancer diagnosis, as well as surgical and survival outcomes (SCORPION [Surgical Complications Related to Primary or Interval debulking in Ovarian Neoplasm] trial NCT01461850, MD Anderson Moon Shot trial, LapOvCa-trial NTR2644).
Laparoscopy has also been used for interval debulking surgery after neoadjuvant therapy. A recent small study comparing 10 patients who underwent laparoscopy and 11 who underwent laparotomy found that laparoscopic interval cytoreduction was feasible with similar recurrence rates. However, the authors recommended that endoscopic surgery for advanced ovarian cancer should be used cautiously because the disease-related mortality rate was higher, and disease-free intervals were shorter in those undergoing endoscopic surgery. Larger prospective trials are needed to determine the safety of endoscopic surgery for advanced ovarian cancer and are currently ongoing (Minimally Invasive Interval Debulking Surgery in Ovarian Neoplasm: a Feasibility Study [MISSION] Trial NCT02324595; Feasibility of Interval Debulking Surgery by Laparoscopy for Peritoneal Carcinosis in Chemosensitive Patients [CILOVE] trial NCT01905163).
In addition, ports for intraperitoneal chemotherapy can be placed with laparoscopic guidance in patients without such access before initiating primary chemotherapy. Last, some investigators have reported on the use of (hand-assisted) laparoscopy as well as robotic surgery for radical intraperitoneal tumor debulking at the time of primary or secondary cytoreductive surgery. These studies lack conclusive data for adequacy of surgery or prospective (randomized) comparison with open technique for morbidity and survival. MIS debulking may be most appropriate for patients requiring a limited number of advanced procedures.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree