Fig. 19.1
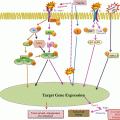
G protein-coupled receptor (GPCR) that is coupled to a Gq heterotrimeric G protein, on binding with the ligand induce activity in the PLC (phospholipase C) isozyme PLC-β, which results in the cleavage of PIP2 into IP3 and DAG. IP3 (also abbreviated Ins3P) is a soluble molecule and is capable of diffusing through the cytoplasm to the ER (endoplasmic reticulum) once it has been produced by the action of PLC. Once at the ER, IP3 is able to bind to the Ins3P receptor (Ins3PR) on a ligand-gated Ca2+ channel that is found on the surface of the ER. The binding of IP3 (the ligand in this case) to IP3R triggers the opening of the Ca2+ channel and thus release of Ca2+ into the cytoplasm. IP3-induced Ca2+ release toward the mitochondria promotes ATP production, inhibition of AMPK and thus stimulation of mTOR activity. Inhibition of mTOR leads to the formation of the ULK1-Atg13-FIP200 complex and autophagy. Ca2+ (and calmodulin) can activate multiple systems in the cell including CaMKKβ which helps in the formation of autophagic complex
19.3 Autophagy, ER Stress and Calcium
A direct link between Ca2+, ER stress, and autophagy relies on the modulation of the inositol 1,4,5-trisphosphate receptor (IP3R). This receptor releases Ca2+ from ER stores in response to different cellular signals, although it could also play additional functions derived from its ability to interact with different proteins including members of the Bcl-2 family. Inhibition of the IP3R with xestospongin B (Criollo et al. 2007) or lithium-induced decrease of myoinositol-1,4,5-triphosphate (IP3) levels (Sarkar et al. 2005) promotes autophagy. Intriguingly, these effects seem to be independent of the Ca2+ mobilization function of IP3R (Criollo et al. 2007). Thus, it has been recently shown that use of pharmacological inhibitors of the IP3R disrupts the interaction of this protein with Beclin-1 (Vicencio et al. 2009) which could be an additional way of regulating the pro-autophagic function of this protein. Further investigation is nevertheless necessary to clarify whether this mechanism participates in the activation of autophagy in response to ER stress.
One recent study suggested that myoinositol-1,4,5-trisphosphate (IP3) could regulate autophagy because inhibition of inositol monophosphatase by lithium or L-690,330 stimulates autophagy through the depletion of inositol and IP3 (Sarkar et al. 2005). IP3 is a second messenger produced primarily in response to the stimulation of G-protein-coupled receptor or receptor tyrosine kinases. IP3 acts on the IP3 receptor (IP3R), a mostly endoplasmic reticulum (ER)-sessile Ca2+ release channel that integrates signals from numerous small molecules and proteins including nucleotides, kinases, and phosphatases, as well as nonenzyme proteins. (Patterson et al. 2004). IP3R is known to play a major role within the Ca2+ microdomains that transmit Ca2+ spikes generated by the ER to mitochondria. (Bianchi et al. 2004). IP3R is also regulated by Bcl-2 (Chen et al. 2004) and Bcl-XL, (White et al. 2005) which affect Ca2+ fluxes through IP3R by direct molecular interactions, by influencing its regulatory phosphorylation and/or by modulating its response to IP3. The process of autophagy induced as a result of stress is to meet the metabolic requirement of the cell. This autophagy has been linked to the inositol triphosphate receptor calcium transfer to mitochondria (Cardenas et al. 2010). The reduced calcium transfer to mitochondria from these calcium channel induces autophagy in HEK cells, MCF-7 and smooth muscle cells through the AMPK sensor pathway (Cardenas et al. 2010). This reduced calcium transfer has also been shown to compromise cell bioenergetics or metabolism in mitochondria effecting flux of nutrients into it.
19.4 Autophagy Can Be Positively or Negatively Regulated by the IP3R
Taken together these results indicate a complex action of the IP3R in autophagy regulation, whereby depending on the state of the cells IP3-induced, Ca2+ release can suppress or promote autophagy. This complex behavior probably also explains in part the contradictory results obtained in cells treated with thapsigargin or BAPTA-AM. Indeed, differing cellular conditions, concentrations of the applied chemicals and incubation times could underlie the different results obtained in different studies (Decuypere et al. 2011). Finally, also the localization of the IP3Rs and the subcellular localization of the resulting Ca2+ signals (cytosol or mitochondria) may determine the specific outcome on autophagy. In addition, it can be expected that regulators of the IP3R may impinge on the cellular autophagy levels by modulating IP3-induced Ca2+ release. Main regulators in this context are obviously Beclin-1 and Bcl-2, though their exact regulation by associated proteins or phosphorylation events remains to be explored. Moreover, as these same proteins control apoptosis (Decuypere et al. 2011), it is extremely important to dissect their relative role and activation mechanisms in both processes.
19.5 Altered IP3R-Expression Profiles in Human Cancers
Different groups have shown the importance of IP3Rs level and/or activity dysregulation in tumor growth, aggressiveness and resistance with a prominent role for IP3R3 (inositol tri phosphate receptor type 3) expression. Regulation of IP3R3 expression by 17β-estradiol (E2) is involved in the growth of the breast cancer epithelial cell line, MCF-7 (Sakaki et al. 2008). E2 triggered Ca2+ release in an IP3R-dependent mechanism, while prolonged E2 exposure led to an increase in IP3R3 expression, which was abrogated by estrogen receptor antagonists. Importantly, knockdown of IP3R3 counteracted the proliferative effect of E2 on MCF-7 cells. IP3R3 expression level is also directly related to aggressiveness of colorectal carcinoma (Lam et al. 2008). Knockdown of IP3R3 in these cancer cells increased apoptosis, while overexpression of IP3R3 decreased apoptosis. High levels of IP3R3 were associated with increased metastasis in the lymph nodes and liver and a decreased 5-year survival. Correlating with these findings, IP3R3 expression was elevated in glioblastoma cells (Grotemeier et al. 2010). Caffeine, which is known to activate ryanodine receptors, paradoxically inhibits IP3R3 activity and blocks glioblastoma invasion (Grotemeier et al. 2010). Also, changes in IP3R1 (inositol tri phosphate receptor type 1) expression have been implicated in the biological properties of tumors. The cisplatin-induced downregulation of IP3R1 expression is associated with the acquisition of cisplatin resistance in bladder cancer cell lines (Wang et al. 2008). Consistent with these findings, IP3R1-knockdown prevented apoptosis and rendered cancer cells more resistant to cisplatin, while overexpression of IP3R1 had the opposite effects. Finally, IP3R-interacting proteins can be targets of chemotherapeutic drugs, thereby affecting IP3R-channel activity. An example is the neuronal Ca2+ sensor 1 (NCS-1), which functions as a taxol-binding protein. Taxol (paclitaxel), used for the treatment of solid tumors, increased the binding of the NCS-1 to the IP3R, potentiating Ca2+ oscillations in these cells. The presence of the Ca2+-binding protein NCS-1 seemed to be essential for taxol effectiveness on cancer (Bootman et al. 2002). Besides differences in total IP3R-expression level, altered IP3R localization should not be excluded in the context of cancer, since cancer-related proteins are involved in proper IP3R subcellular localization.
Conclusion
IP3Rs are critical Ca2+-signaling hubs that critically control cell survival, adaptation, death processes, and themselves are tightly regulated by proto-oncogenes and tumor-suppressor proteins (Akl and Bultynck 2013). Altered IP3R activity and/or the remodeling of IP3R-expression profiles may be exploited by cancer cells to promote their survival, growth, proliferation and migration. Altered IP3R protein complex formation can affect mitochondrial bioenergetics and susceptibility to autophagic stimuli, thereby enabling the survival or death of cells with oncogenic features. Understanding the detailed molecular regulation of IP3Rs will be important to develop novel therapeutic strategies to target cancer cells through their deregulated Ca2+-signaling machinery.
References
Akl H, Bultynck G (2013) Altered Ca(2+) signaling in cancer cells: proto-oncogenes and tumor suppressors targeting IP3 receptors. Biochim Biophys Acta 1835(2):180–193
Beugnet A, Tee AR, Taylor PM, Proud CG (2003) Regulation of targets of mTOR (mammalian target of rapamycin) signalling by intracellular amino acid availability. Biochem J 372:555–566PubMedCentralPubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree