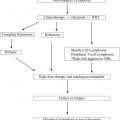
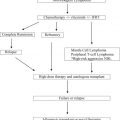
Myeloproliferative neoplasms (MPN) are clonal hematopoietic stem cell disorders. While some MPN patients have an indolent course, all are at risk of progressing to severe marrow failure or transforming into acute leukemia. Allogeneic hematopoietic cell transplantation (allo-HCT) is the only potential curative therapy. Major pre-transplant risk factors are disease stage of the MPN, the presence of comorbid conditions and the use of HLA non-identical donors. The development of reduced-intensity conditioning regimens has allowed for successful allo-HCT even for older patients and patients with comorbid conditions. The pre-transplant use of JAK2 inhibitors, which may be effective in down staging a patient’s disease, may improve the outcomes following allo-HCT.
Key points
- •
Allogeneic stem cell transplantation remains the only curative therapy for Myeloproliferative neoplasms.
- •
Transplantation following reduced-intensity conditioning provides survival equivalent to that with high-intensity (myeloablative) regimens.
- •
Alternative stem cell sources, including umbilical cord blood, are acceptable for patients without HLA-matched siblings.
- •
Janus kinase 2 inhibitors (ruxolitinib) may provide beneficial adjuvant therapy in patients undergoing allogeneic hematopoietic cell transplantation.
Introduction
Myelofibrosis (MF) can present as de novo primary MF (PMF) or evolve from other myeloproliferative neoplasms (MPN), such as polycythemia vera (PV) or essential thrombocythemia (ET). Regardless of the cause, MF is characterized as a clonal stem cell disorder associated with dysregulated Janus kinase (JAK)/signal transducers and activators of transcription signaling and elevated levels of proinflammatory and proangiogenic cytokines, such as tumor necrosis factor alpha, interleukin 6, and interferon gamma, resulting in a bone marrow stromal reaction that includes varying degrees of reticulin and collagen fibrosis and osteosclerosis. Clinically, MF is typified by progressive anemia, leukopenia or leukocytosis, thrombocytopenia or thrombocythemia, and multi-organ extramedullary hematopoiesis. It frequently involves the spleen, resulting in massive splenomegaly, severe constitutional symptoms, a hypermetabolic state, and cachexia. The median age at diagnosis is 60 to 65 years. The clinical course is heterogeneous, ranging from indolent disease and survival for decades to aggressive disease in other cases, with survival measured in months. The most common causes of death are progressive marrow failure leading to infection or hemorrhage, transformation to acute myelogenous leukemia, and complications of portal hypertension.
Introduction
Myelofibrosis (MF) can present as de novo primary MF (PMF) or evolve from other myeloproliferative neoplasms (MPN), such as polycythemia vera (PV) or essential thrombocythemia (ET). Regardless of the cause, MF is characterized as a clonal stem cell disorder associated with dysregulated Janus kinase (JAK)/signal transducers and activators of transcription signaling and elevated levels of proinflammatory and proangiogenic cytokines, such as tumor necrosis factor alpha, interleukin 6, and interferon gamma, resulting in a bone marrow stromal reaction that includes varying degrees of reticulin and collagen fibrosis and osteosclerosis. Clinically, MF is typified by progressive anemia, leukopenia or leukocytosis, thrombocytopenia or thrombocythemia, and multi-organ extramedullary hematopoiesis. It frequently involves the spleen, resulting in massive splenomegaly, severe constitutional symptoms, a hypermetabolic state, and cachexia. The median age at diagnosis is 60 to 65 years. The clinical course is heterogeneous, ranging from indolent disease and survival for decades to aggressive disease in other cases, with survival measured in months. The most common causes of death are progressive marrow failure leading to infection or hemorrhage, transformation to acute myelogenous leukemia, and complications of portal hypertension.
Patient evaluation overview
Prognosis of MF varies with the presence or absence of specific risk factors. Historical prognostic scoring systems, such as the Lille or Dupriez classification, focused primarily on blood cell counts as the major prognostic factor. In 2009, Cervantes and colleagues published a multicenter analysis of risk factors and their impact on prognosis in patients with MF. Age greater than 65 years; presence of constitutional symptoms, including weight loss, fever, or night sweats, anemia (hemoglobin [Hb] <10 g/dL), leukocytosis (white blood cells [WBC] greater than 25 × 109/L); and a circulating blast percentage of 1% or greater were identified as most predictive of outcome. Median survival among patients with no risk factors (low-risk group) was 135 months, compared with 95 months among patients with one risk factor (intermediate 1 risk), 48 months among those with 2 risk factors (intermediate 2 risk), and 27 months among those with 3 or more risk factors (high risk). This risk stratification is referred to as the international prognostic scoring system (IPSS) ( Table 1 ).
| Variable | IPSS | DIPSS | DIPSS-Plus |
| Age >65 y | X | X | X |
| Constitutional symptoms | X | X | X |
| Hb <100 g/L | X | X | X |
| WBC >25 × 10 9 /L | X | X | X |
| Circulating blasts >1% | X | X | X |
| Platelets <100 × 10 9 /L | — | — | X |
| RBC transfusion need | — | — | X |
| Unfavorable karyotype | — | — | X |
| 1 Point each | 1 Point each but Hb = 2 | 1 Point each |
The Dynamic IPSS (DIPSS), which was subsequently developed, uses the same 5 variables but can be used at any time in the disease course. However, unlike the IPSS, Hb less than 10 g/L receives a score of 2 points. The risk groups are scored as follows: low risk (score = 0), intermediate 1 (score = 1), intermediate 2 (score = 2 or 3), or high risk (score = 4–6) with corresponding median survivals of 185, 78, 35, and 16 months, respectively. The DIPSS plus scoring system includes an additional 3 risk factors: transfusion dependence, unfavorable cytogenetics (+8, -7/7q-, i [17q], inversion [inv] [3], -5, 5q-, 12p-, 11q23, and complex karyotype), and platelet count less than 100 × 10 9 /L.
Indications for transplantation
IPSS, DIPPS, or DIPPS-plus risk classifications, comorbidities, and donor availability should be used to identify patients who are candidates for allogeneic stem cell transplantation (allo-SCT). Current recommendations imply that the potential risk of transplant-related complications is justified in transplant-eligible patients with less than 5 years expected survival if not transplanted. Based on DIPSS data, this would include patients in the intermediate 2– and high-risk groups. Those patients are, indeed, the patients included in most clinical transplant trials; however, lower-risk patients actually have a better outcome following allogeneic hematopoietic cell transplantation (allo-HCT), raising questions as to the most appropriate selection of patients and timing for allo-HCT. Published data from the Fred Hutchinson Cancer Research Center (FHCRC) on 170 patients, with a median age of 51.5 years, with MF showed that the 6-year post–allo-HCT survival for low, intermediate 1–, intermediate 2–, and high-risk patients was 80%, 67%, 54%, and 38%, respectively, validating the DIPSS as an accurate prognosticator for post–allo-HCT outcomes in MF.
Tefferi and colleagues recently completed an analysis of 884 patients aimed at identifying risk factors associated with a greater than 80% 2-year mortality. Poor prognostic features included monosomal karyotype, inv (3)/i (17q) abnormalities, or any 2 of the following factors: peripheral blast percentage greater than 9%, WBC greater than 40 × 10 9 /L, or other unfavorable karyotypes. Those patients meeting the performance status and donor criteria for allo-HCT should be referred directly to allo-HCT rather than undergo conventional treatment. In another study, which characterized 793 patients with PMF using DIPSS-plus criteria, the only 2 risk factors for leukemic transformation were unfavorable karyotype and platelet count less than 100 × 10 9 /L; the 10-year risk of leukemic transformation was 12% in the absence of these 2 risk factors and 31% in the presence of one or both risk factors. As is becoming evident for overall survival (OS), leukemia-free survival is also significantly compromised in patients carrying certain mutations, including ASXL1, IDH1 / 2 , EZH2 , and SRSF2 .
Transformation into acute leukemia occurs in 10% to 20% of patients and is associated with a median 5-month survival. Previous studies have shown that postponing transplantation in MF until patients are at a more advanced stage of disease resulted in worse outcome. In a study performed by the MPN subcommittee of the Chronic Myeloid Leukemia Working Party of the European Bone Marrow Transplant (EBMT) group looking at 46 patients with leukemic transformation of PMF or post–ET/PV MF, 3-year OS, and progression-free survival (PFS) were 33% and 26%, respectively. Only remission status was significantly predictive for OS and PFS (69% vs 22%; P = .0008). However, the survival benefit of patients transplanted in complete remission (CR) was caused by significantly lower treatment-related mortality (TRM) while relapse incidence was identical. The consensus by the EBMT/european leukemia net (ELN) working committee in 2014 is that patients with leukemic transformation should receive cytoreductive therapy and be considered for transplantation only after achieving a partial or CR of leukemia.
Timing of and preparing patients for transplant
Although allo-HCT remains the only known curative therapy for MF, any treatment that improves the patients’ performance status and reduces the individual transplant-specific risk should be considered in the pretransplant phase. In the past, drug therapies, including hydroxyurea, busulfan, 6-mercaptopurine, anagrelide, thalidomide, lenalidomide, interferon, corticosteroids, androgens, and erythropoiesis-stimulating agents or other growth factors, were used as the first-line treatment of MF. These treatments may be helpful for palliation of symptoms; but responses are typically of short duration, usually less than 1 year. The Food and Drug Administration (FDA) recently approved ruxolitinib, a selective JAK-1/2 inhibitor, for the treatment of primary and secondary MF. JAK2 inhibitor therapy (ruxolitinib or others) is indicated in patients with a spleen extending more than 5 cm from the left costal margin and patients with constitutional symptoms. The drug should be administered for a minimum of 1 month but tapered off before transplant conditioning. Impressive symptom control in patients with MF on ruxolitinib treatment is predominantly mediated by profound suppression of proinflammatory and proangiogenic cytokines. Reduction of cytokine levels is typically accompanied by a substantial decrease in spleen size. Patients should be treated to best response.
The potential benefits of (surgical) splenectomy are a matter of debate. Data from Mayo Clinic on 314 patients with MF undergoing splenectomy showed significant perioperative complications in 28% of patients and a mortality of 6.7%. The report by Ballen and colleagues in 2010 on 289 patients with MF failed to show a significant effect of prior splenectomy on graft failure or PFS. Ciurea in 2008 noted that successful engraftment still was achieved even with massive splenomegaly. Michallet and colleagues showed in 2009 greater severity of graft-versus-host disease (GVHD) in splenectomized patients. A recent report from the MD Anderson Cancer Center demonstrated improvement in symptoms, such as anemia and thrombocytopenia, as well as abdominal discomfort at the cost of increased TRM. However, there are also proponents for pre–allo-HCT splenectomy in patients with MF. Li and colleagues showed in 2001 that splenectomized patients had faster neutrophil recovery and decreased transfusion requirements. Robin and colleagues, in a multivariate analysis of results in patients receiving peripheral blood stem cells, showed faster engraftment in patients without splenomegaly and those who had undergone splenectomy pretransplant. Kroger and colleagues demonstrated a trend toward more rapid neutrophil engraftment in splenectomized versus nonsplenectomized patients with reduced-intensity conditioning (RIC) allo-HCT. However, in that study a higher rate of relapse at 3 years was seen in splenectomized patients. On the other hand, the retrospective analysis by Scott and colleagues in 170 patients transplanted at the FHCRC, suggests that splenectomized patients had superior post–allo-HCT survival ( P = .05). Bacigalupo and colleagues suggested that a spleen size greater than 22 cm was an independent risk factor for survival.
In summary, for patients with refractory, symptomatic splenomegaly, the evidence in support of splenectomy to improve transplant outcome is not sufficient to recommend splenectomy as a standard pretransplant procedure. Pretransplant splenectomy in patients with refractory splenomegaly should be decided on an individual basis. If this procedure is performed, it should be in the setting of a clinical trial and at a center that performs many procedures per year and has a proven track record of success.
Autologous Stem Cell Transplant
Data on autologous SCT for MPN are sparse. In a multicenter study, 27 patients with MF in the setting of PMF, PV, or ET underwent autologous hematopoietic cell collection and 21 underwent conditioning with busulfan only, followed by autologous HCT. The median time to platelet and neutrophil recovery was 21 days (for both), with clinically significant responses seen in 10 of 17 patients with anemia, 4 of 8 patients with thrombocytopenia, and 7 of 10 patients with symptomatic splenomegaly. However, the study was closed because of graft failure or incomplete hematopoietic recovery in 5 patients (27%). The high graft failure rate was attributed to the fact that autologous hematopoietic cells had been collected late in the disease course, and the investigators speculated that engraftment might be improved if autologous cells had been harvested earlier. The benefit achieved with autologous cells was thought to be caused by the faster growth of normal stem cells than that of clonal malignant cells. To carry out autologous HCT for MPN with curative intent, purging of clonal hematopoietic cells from the harvested cells would be necessary; currently, no effective method to achieve this objective is available.
Allogeneic Stem Cell Transplant
Allo-HCT remains the only curative therapy for MF. Initial trials at the FHCRC showed that patients with severe marrow fibrosis were prone to experience engraftment failure (33% in a cohort of 15 patients), whereas engraftment was prompt in patients with mild or moderate fibrosis (6% failure in a cohort of 32 patients). However, subsequently patients with severe MF or even osteosclerosis were shown to achieve sustained engraftment and regression or even complete resolution of marrow fibrosis with normalization of the marrow architecture following allo-HCT.
Myeloablative Conditioning
Although a broad range of conditioning regimens of different intensities has been developed, the initial studies of allo-HCT for MF used high-intensity myeloablative conditioning (MAC) regimens, usually including total body irradiation (TBI) or busulfan. For MAC regimens, graft failure rates of less than 5% to 30% have been reported, reflecting the heterogeneity in patient populations and specific conditioning regimens. Published TRM rates of 10% to 35% at 1 year and OS from 30% to 67% at 5 years have been reported ( Table 2 ).
| Study | N | Conditioning | Age (y) | 1-y TRM (%) | 5-y OS (%) | 5-y PFS (%) |
|---|---|---|---|---|---|---|
| Ballen et al, 2010 | 229 | MAC | 47 | 27 (Sib) | 37 (Sib) | 33 (Sib) |
| 43 (URD) | 30 (URD) | 27 (URD) | ||||
| Patriarca et al, 2008 | 100 | MAC | 49 | 35 | 31 | 28 |
| Kerbauy et al, 2007 | 104 | MAC | 49 | 27 | 61 (7 y) | — |
| Deeg et al, 2003 | 56 | MAC | 43 | 35 | 58 | — |
| Guardiola et al, 1999 | 55 | MAC | 42 | 27 | 47 | — |
| Abelsson et al, 2012 | 40 | MAC | 46 | — | 49 | — |
| 52 | RIC | 55 | 59 | |||
| Robin et al, 2011 | 46 101 | MAC RIC | 53 | 29 (4 y) | 39 (4 y) | 32 (4 y) |
| Stewart et al, 2010 | 27 | MAC | 38 | 41 (3 y) | 44 (3 y) | 44 (3 y) |
| 24 | RIC | 54 | 32 (3 y) | 31 (3 y) | 24 (3 y) | |
| Kroger et al, 2009 | 103 | RIC | 53 | 16 | 67 | 51 |
| Ballen et al, 2010 | 60 | RIC | — | 15 | — | 39 |
| Bacigalupo et al, 2010 | 46 | RIC | 51 | 24 | 45 | 43 |
| Alchaby et al, 2012 | 150 | RIC | 57 | — | 60 | — |
| Rondelli et al, 2005 | 21 | RIC | 54 | 10 | 85 (2.5 y) | — |
| Gupta et al, 2014 | 233 | RIC | 55 | 24 (5 y) | 47 | 27 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree