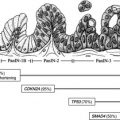
Accurate diagnosis and staging of pancreatic neoplasms is essential for surgical planning and identification of locally advanced and metastatic disease that is incurable by surgery. The ability to position the endoscopic ultrasonography (EUS) transducer close to the pancreas combined with the use of fine-needle aspiration enables the accurate diagnosis of pancreatic cysts and solid masses. EUS is also increasingly being used to procure core tissue for molecular analysis that facilitates personalized treatment of pancreatic cancer. Various therapeutic interventions can be undertaken under EUS guidance. This article focuses on the applications of EUS and endoscopic retrograde cholangiopancreatography in pancreatic neoplasms.
Key points
- •
Endoscopic ultrasonography (EUS)–guided fine-needle aspiration is an accurate technique for establishing tissue diagnosis in patients with pancreatic mass lesions.
- •
There has been growing interest in evaluating core tissue specimens procured by EUS for molecular markers that may serve as prognostic predictors and targets for focused therapy in pancreatic cancer.
- •
Endoscopic retrograde cholangiopancreatography (ERCP) with biliary stent placement relieves obstructive jaundice and is widely practiced both as a palliative measure and for preoperative biliary decompression in pancreatic cancer.
- •
EUS-guided drainage is becoming an effective rescue therapy for relief of obstructive jaundice in patients who fail ERCP, and it may be superior to percutaneous transhepatic biliary drainage.
Endoscopic ultrasonography in pancreatic neoplasms
Endoscopic ultrasonography (EUS) is a sensitive technology for detecting pancreatic lesions and for performing fine-needle aspiration (FNA). Although computed tomography (CT) and magnetic resonance cholangiopancreatography are excellent modalities for imaging the pancreas, neuroendocrine tumors and pancreatic cyst lesions are better characterized by EUS. The diagnostic sensitivity of EUS-guided FNA (EUS-FNA) exceeds 85% to 90% and it is significantly superior to percutaneous techniques. In addition, EUS enables additional interventions, such as celiac plexus neurolysis (CPN) for palliation of pain, fiducial placements to facilitate intraoperative identification of small tumors or improved targeting of image-guided radiation therapy, and drainage of well-encapsulated pancreatic fluid collections after a distal pancreatectomy. The diagnostic and therapeutic applications of EUS in pancreatic neoplasms are discussed here.
Pancreatic Cancer
Staging endoscopic ultrasonography
Staging of pancreatic malignancy is performed according to the American Joint Committee for Cancer Staging TNM (tumor, node, metastasis) classification. Reported accuracies of EUS range from 63% to 94% for T classification and 41% to 86% for N classification. Although early studies found that EUS was superior to conventional CT for T and N staging, recent studies report that EUS is not superior to CT and MRI for T and N staging of pancreatic tumors. The initial advantages shown by EUS compared with the other imaging modalities for staging of pancreatic tumors were not confirmed in subsequent studies.
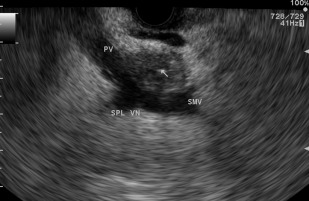
The sensitivity and specificity of EUS for malignant vascular invasion are 42% to 91% and 89% to 100%, respectively. Although some studies show that EUS is more accurate than CT for vascular invasion, other investigators have reported that the accuracy of CT is superior to that of EUS. The overall accuracy of MRI is reportedly equivalent or superior to that of EUS. The overall sensitivity and accuracy of EUS for arterial invasion are 56% and 50%, respectively. The sensitivity of EUS for tumor invasion of the portal vein or portal vein confluence is 60% to 100%, with most studies showing sensitivities of more than 80% ( Fig. 1 ). The sensitivity of EUS for portal vein invasion is also consistently superior to that of CT. For the superior mesenteric vein (SMV), superior mesenteric artery (SMA), and celiac artery, the sensitivity of EUS is only 17% to 83%, 17%, and 50%, respectively. The sensitivity and specificity of EUS for determining resectability of pancreatic cancer in a pooled analysis of 377 patients was 69% (range, 23%–91%) and 82% (63%–100%), respectively. The overall EUS accuracy for tumor resectability was reported at 77%. Thus, more cost and decision analysis and comparative studies of EUS with state-of-the-art CT and MRI are required to make definitive recommendations on the precise role of EUS in staging pancreatic cancer.
Endoscopic ultrasonography fine-needle aspiration
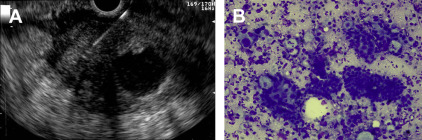
Two recently published meta-analyses totaling more than 8400 patients and 67 studies reported pooled sensitivities for the diagnosis of malignancy based on cytology of 85% and 89% and pooled specificities of 98% and 99%. However, the negative predictive value of EUS-FNA for pancreatic tumors remains limited at 65%. Therefore a negative or nondiagnostic biopsy does not exclude the possibility of malignancy. Fritscher-Ravens and colleagues found that, in a series of 207 consecutive patients with focal pancreatic lesions, the sensitivity of EUS-FNA for the diagnosis of malignancy in patients with normal parenchyma (89%) was superior to the sensitivity in those with chronic pancreatitis (54%). The presence of chronic pancreatitis may also hinder cytologic interpretation of pancreatic biopsy, thus decreasing the sensitivity of EUS-FNA of pancreatic masses. In a meta-analysis of 34 studies that evaluated the diagnostic accuracy of EUS-FNA for pancreatic cancer in 3644 patients with solid pancreatic mass lesions, rapid on-site evaluation (ROSE) was a significant determinant of EUS-FNA accuracy. Also, at least 5 to 7 EUS-FNA passes should be performed when sampling pancreatic masses to maximize diagnostic yield ( Fig. 2 ) and this information may prove helpful to endosonographers performing EUS-FNA when ROSE is not available.
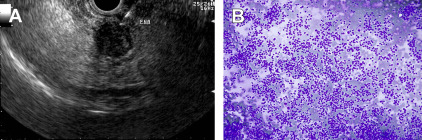
Occasionally, ROSE may be inconclusive because of tumor necrosis, fibrosis, or hypervascularity. The diagnostic yield may be increased by fanning the lesion, in which different angles of scope deflection are used in order to sample the peripheral parts of the lesion. In a recent randomized study by these authors, the fanning technique was superior to the standard approach, with fewer passes being required to establish a diagnosis.
The most commonly used commercially available EUS-FNA needle sizes are 19, 22, and 25 gauge. In a recent meta-analysis of 8 studies comprising 1292 patients, 25-gauge needles were associated with higher sensitivity but comparable specificity with 22-gauge needles. In general, 25-gauge needles are associated with lower technical failures compared with 19-gauge and 22-gauge needles when sampling pancreatic head and uncinate process lesions.
Postprocedure adverse events following EUS-FNA are rare. In a recent systematic review that included 8246 patients with pancreatic lesions, 7337 of those being solid masses, minor adverse events after EUS-FNA occurred in 60 patients (0.82%), which included pancreatitis, abdominal pain, bleeding, fever, and infection.
Pancreatic Neuroendocrine Tumors
Pancreatic neuroendocrine tumors (PNETs) represent less than 10% of pancreatic tumors. In a series of studies that compared EUS with other imaging modalities, the sensitivity of EUS for detection of PNETs was 77% to 94%. EUS seems especially useful for detection of small PNETs (<2.5 cm) missed by other imaging studies. In a study of 30 patients with 32 insulinomas, the sensitivity of EUS was 94% compared with 29% for nonhelical CT and 57% for dual-phase multidetector CT. In another study of 217 patients, CT was more likely than EUS to miss insulinomas and PNETs less than 2 cm. These investigators also found that the overall sensitivity of EUS (91%) was greater than that of CT (63%). In recent studies, the reported sensitivity of MRI for PNET detection is 85% to 100% with positive predictive value of 96%.
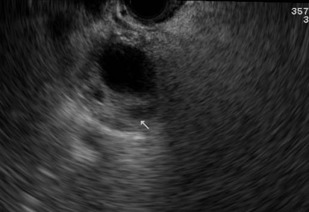
In a series of patients with histologically proven insulinomas (n = 20) or gastrinomas (n = 21), the sensitivity and positive predictive value of EUS were 77% and 94%, respectively. For the same patients, the sensitivity and positive predictive value of somatostatin receptor scintigraphy (SRS) for insulinoma and gastrinoma detection were 60% and 100%, and 25% and 100%, respectively. When both tests were combined and a subgroup analysis was performed, the overall sensitivity of combined EUS and SRS was 89% for insulinomas and 93% for gastrinomas. It seems that the combination of EUS and SRS may optimize preoperative identification of PNETs and limit the need for more invasive tests such as angiography. As with pancreatic cancer, EUS-FNA seems to be an excellent modality for establishing tissue diagnosis in PNETs ( Fig. 3 ). In a recent meta-analysis of 13 studies comprising 456 patients, the pooled sensitivity of EUS for detecting PNET was 87% and the pooled specificity was 98%.
Pancreatic Cyst Lesions
When a cystic lesion has been identified in the pancreas, the size, location, relation to adjacent vessels and organs, and the presence of locoregional or distant metastases should be noted. The cyst should be examined to determine the wall thickness, presence of a mural nodule, or associated mass. The size of the main pancreatic duct, whether it communicates with the cyst, the presence of mucin or a mural nodule within the pancreatic duct, or any focal dilatation should also be noted.
There are several features on EUS that are worrisome for malignant transformation of the cyst and these include thick wall or septum, an associated solid mass, or a mural nodule ( Fig. 4 ). The presence of focal dilatation of the main pancreatic duct, pancreatic duct measuring greater than or equal to 10 mm, or mural nodules are features associated with malignant transformation (See Greer JB, Ferrone CR: Spectrum and classification of cystic neoplasms of the pancreas , in this issue).
The risk of adverse events from EUS-FNA of pancreatic cysts is slightly higher than that resulting from EUS-FNA of a solid lesion. In a recent review of adverse events associated with EUS-FNA, the most common adverse event was pancreatitis, which occurred in 1.1% of patients, followed by pain (0.77%), bleeding (0.33%), fever (0.33%), and infection (0.22%). Antibiotic prophylaxis is therefore recommended, usually with intravenous ciprofloxacin before the procedure. If FNA is performed, the aspirate must be sent for carcinoembryonic antigen (CEA) and/or cytology.
The specificity of cytology for malignancy is excellent and approaches 100%, but the sensitivity varies considerably in reported series. This finding reflects the difficulty in interpreting these lesions, especially when the cellularity of the samples is low. Brandwein and colleagues and Brugge and colleagues reported sensitivities of 55% and 59%, respectively, for differentiating benign from malignant or potentially malignant pancreatic cysts. The CEA levels are higher in mucinous than in nonmucinous cysts, with a reference value of 192 ng/mL for mucinous cysts. In a large study, the diagnostic accuracy of this reference level for differentiating mucinous from nonmucinous cysts was 79%. Although molecular markers such as KRAS and GNAS are being studied as indicators of malignant transformation, data are limited and further research is needed before they can be incorporated into clinical practice.
The Concept of Core Biopsy
Although considerable progress has been made in the treatment of breast and lung cancers in which the delivery of chemotherapy is guided by the expression of molecular markers in the tumor, which can in turn help prognosticate the tumor and guide treatment algorithms, data on personalized therapy in pancreatic cancer are scant. There is growing evidence that immune cells in pancreatic ductal adenocarcinoma produce immune suppressive signals that allow tumors to evade the immune response. Also, the stromal fibroblasts provide a protective environment that not only supports and promotes pancreatic adenocarcinoma tumor growth and progression but also likely suppresses the development of and/or access to the antitumor immune responses. Strategies to deplete the desmoplastic stroma before institution of immune therapy could promote robust response against tumor cells. Therefore, it is increasingly apparent that the evaluation of fibrous stroma for molecular markers may be an integral part of cancer therapy. Preliminary evidence in our laboratory suggests that specimens procured using a 19-gauge or 22-gauge FNA needle and fixed in formalin have preserved microcores that enable detailed assessment of the histologic architecture with neoplastic glands embedded in the stroma. Randomized trials are in progress to identify the best technique for core tissue procurement. We believe that the results of these trials will be of significant help in advancing personalized therapy in pancreatic cancer.
Therapeutic Applications of Endoscopic Ultrasonography in Pancreatic Neoplasms
Endoscopic ultrasonography–guided celiac plexus neurolysis
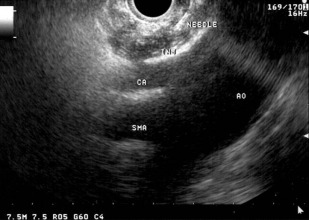
At EUS, after identifying the celiac artery take-off from the aorta, the FNA needle is positioned adjacent and anterior to the lateral aspect of the aorta at the level of the celiac trunk ( Fig. 5 ). The FNA needle is then aspirated to rule out vessel penetration before injection. For CPN in patients with pancreatic cancer, 10 mL of 0.25% bupivacaine are injected followed by 10 mL of dehydrated (98%) alcohol. A recent meta-analysis reported that 80% of patients with pancreatic cancer undergoing CPN had pain relief. Also, patients who had injections on both sides of the celiac artery had a higher rate of pain relief than those who received injections only on 1 side (85% vs 46%). In a randomized trial, 96 patients with inoperable pancreatic cancer were assigned to either CPN or conventional pain management. At 3 months, patients treated with CPN had significantly greater pain relief, with a trend toward lower morphine consumption. There was no difference between the two groups in quality-of-life scores or survival.
Endoscopic ultrasonography–guided implantation therapy
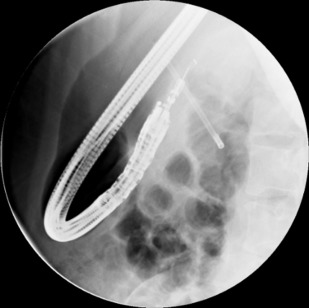
PNETs are small and tumor localization at laparoscopic surgery can be challenging. EUS-guided fiducial placement is a new technique for intraoperative localization of pancreatic tumors ( Fig. 6 ). At EUS, once a tumor is localized, a small gold fiducial (2–3 mm × 0.8 mm) is back-loaded to a 19-gauge FNA needle (after stylet retraction) and the lumen of the needle is sealed with bone wax. After puncturing the lesion, the stylet is advanced to facilitate deployment of gold fiducials within the tumor. At surgery, using fluoroscopy, the tumor is identified to facilitate laparoscopic resection. Likewise, fiducial placement enables better targeting of pancreatic cancers by image-guided radiation therapy.
Endoscopic ultrasonography–guided pancreatic cyst ablation
Ethanol and chemotherapeutic agents such as paclitaxel are used for EUS-guided ablation of pancreatic cyst lesions under EUS guidance. In a systematic review, the pooled proportion of patients with complete cyst resolution was 56.2% (95% confidence interval [CI], 48.2%–64.1%) and partial cyst resolution was 23.7% (95% CI, 17.2%–30.9%). Postprocedural adverse events included abdominal pain in 6.5% (95% CI, 3.1%–11.0%) and pancreatitis in 3.9% (95% CI, 1.4–7.6).
Endoscopic ultrasonography–guided drainage of postoperative pancreatic fluid collections
Development of a postoperative pancreatic fluid collection is a common complication after distal pancreatectomy and is usually managed by percutaneous drainage. Major disadvantages of percutaneous drainage include fistula formation, infection, skin excoriation, and accidental dislodging of the catheter. At EUS, the pancreatic fluid collection can be easily accessed using a 19-gauge needle. After dilating the transmural tract to 6 to 10 mm, multiple internal stents can be deployed for transgastric drainage ( Fig. 7 ). The treatment success rate for this approach exceeds 90% and it yields faster symptom relief and resolution of the pancreatic fluid collection compared with percutaneous drainage or transpapillary stenting.
Endoscopic ultrasonography in pancreatic neoplasms
Endoscopic ultrasonography (EUS) is a sensitive technology for detecting pancreatic lesions and for performing fine-needle aspiration (FNA). Although computed tomography (CT) and magnetic resonance cholangiopancreatography are excellent modalities for imaging the pancreas, neuroendocrine tumors and pancreatic cyst lesions are better characterized by EUS. The diagnostic sensitivity of EUS-guided FNA (EUS-FNA) exceeds 85% to 90% and it is significantly superior to percutaneous techniques. In addition, EUS enables additional interventions, such as celiac plexus neurolysis (CPN) for palliation of pain, fiducial placements to facilitate intraoperative identification of small tumors or improved targeting of image-guided radiation therapy, and drainage of well-encapsulated pancreatic fluid collections after a distal pancreatectomy. The diagnostic and therapeutic applications of EUS in pancreatic neoplasms are discussed here.
Pancreatic Cancer
Staging endoscopic ultrasonography
Staging of pancreatic malignancy is performed according to the American Joint Committee for Cancer Staging TNM (tumor, node, metastasis) classification. Reported accuracies of EUS range from 63% to 94% for T classification and 41% to 86% for N classification. Although early studies found that EUS was superior to conventional CT for T and N staging, recent studies report that EUS is not superior to CT and MRI for T and N staging of pancreatic tumors. The initial advantages shown by EUS compared with the other imaging modalities for staging of pancreatic tumors were not confirmed in subsequent studies.
The sensitivity and specificity of EUS for malignant vascular invasion are 42% to 91% and 89% to 100%, respectively. Although some studies show that EUS is more accurate than CT for vascular invasion, other investigators have reported that the accuracy of CT is superior to that of EUS. The overall accuracy of MRI is reportedly equivalent or superior to that of EUS. The overall sensitivity and accuracy of EUS for arterial invasion are 56% and 50%, respectively. The sensitivity of EUS for tumor invasion of the portal vein or portal vein confluence is 60% to 100%, with most studies showing sensitivities of more than 80% ( Fig. 1 ). The sensitivity of EUS for portal vein invasion is also consistently superior to that of CT. For the superior mesenteric vein (SMV), superior mesenteric artery (SMA), and celiac artery, the sensitivity of EUS is only 17% to 83%, 17%, and 50%, respectively. The sensitivity and specificity of EUS for determining resectability of pancreatic cancer in a pooled analysis of 377 patients was 69% (range, 23%–91%) and 82% (63%–100%), respectively. The overall EUS accuracy for tumor resectability was reported at 77%. Thus, more cost and decision analysis and comparative studies of EUS with state-of-the-art CT and MRI are required to make definitive recommendations on the precise role of EUS in staging pancreatic cancer.
Endoscopic ultrasonography fine-needle aspiration
Two recently published meta-analyses totaling more than 8400 patients and 67 studies reported pooled sensitivities for the diagnosis of malignancy based on cytology of 85% and 89% and pooled specificities of 98% and 99%. However, the negative predictive value of EUS-FNA for pancreatic tumors remains limited at 65%. Therefore a negative or nondiagnostic biopsy does not exclude the possibility of malignancy. Fritscher-Ravens and colleagues found that, in a series of 207 consecutive patients with focal pancreatic lesions, the sensitivity of EUS-FNA for the diagnosis of malignancy in patients with normal parenchyma (89%) was superior to the sensitivity in those with chronic pancreatitis (54%). The presence of chronic pancreatitis may also hinder cytologic interpretation of pancreatic biopsy, thus decreasing the sensitivity of EUS-FNA of pancreatic masses. In a meta-analysis of 34 studies that evaluated the diagnostic accuracy of EUS-FNA for pancreatic cancer in 3644 patients with solid pancreatic mass lesions, rapid on-site evaluation (ROSE) was a significant determinant of EUS-FNA accuracy. Also, at least 5 to 7 EUS-FNA passes should be performed when sampling pancreatic masses to maximize diagnostic yield ( Fig. 2 ) and this information may prove helpful to endosonographers performing EUS-FNA when ROSE is not available.
Occasionally, ROSE may be inconclusive because of tumor necrosis, fibrosis, or hypervascularity. The diagnostic yield may be increased by fanning the lesion, in which different angles of scope deflection are used in order to sample the peripheral parts of the lesion. In a recent randomized study by these authors, the fanning technique was superior to the standard approach, with fewer passes being required to establish a diagnosis.
The most commonly used commercially available EUS-FNA needle sizes are 19, 22, and 25 gauge. In a recent meta-analysis of 8 studies comprising 1292 patients, 25-gauge needles were associated with higher sensitivity but comparable specificity with 22-gauge needles. In general, 25-gauge needles are associated with lower technical failures compared with 19-gauge and 22-gauge needles when sampling pancreatic head and uncinate process lesions.
Postprocedure adverse events following EUS-FNA are rare. In a recent systematic review that included 8246 patients with pancreatic lesions, 7337 of those being solid masses, minor adverse events after EUS-FNA occurred in 60 patients (0.82%), which included pancreatitis, abdominal pain, bleeding, fever, and infection.
Pancreatic Neuroendocrine Tumors
Pancreatic neuroendocrine tumors (PNETs) represent less than 10% of pancreatic tumors. In a series of studies that compared EUS with other imaging modalities, the sensitivity of EUS for detection of PNETs was 77% to 94%. EUS seems especially useful for detection of small PNETs (<2.5 cm) missed by other imaging studies. In a study of 30 patients with 32 insulinomas, the sensitivity of EUS was 94% compared with 29% for nonhelical CT and 57% for dual-phase multidetector CT. In another study of 217 patients, CT was more likely than EUS to miss insulinomas and PNETs less than 2 cm. These investigators also found that the overall sensitivity of EUS (91%) was greater than that of CT (63%). In recent studies, the reported sensitivity of MRI for PNET detection is 85% to 100% with positive predictive value of 96%.
In a series of patients with histologically proven insulinomas (n = 20) or gastrinomas (n = 21), the sensitivity and positive predictive value of EUS were 77% and 94%, respectively. For the same patients, the sensitivity and positive predictive value of somatostatin receptor scintigraphy (SRS) for insulinoma and gastrinoma detection were 60% and 100%, and 25% and 100%, respectively. When both tests were combined and a subgroup analysis was performed, the overall sensitivity of combined EUS and SRS was 89% for insulinomas and 93% for gastrinomas. It seems that the combination of EUS and SRS may optimize preoperative identification of PNETs and limit the need for more invasive tests such as angiography. As with pancreatic cancer, EUS-FNA seems to be an excellent modality for establishing tissue diagnosis in PNETs ( Fig. 3 ). In a recent meta-analysis of 13 studies comprising 456 patients, the pooled sensitivity of EUS for detecting PNET was 87% and the pooled specificity was 98%.