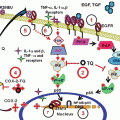
Fig. 1
Schematic overview of CXCR4-SDF-1α-induced intracellular signal transduction pathway and desensitization of activated receptor signaling
Since SDF-1α is constitutively expressed, it has been reported that shutting off continuous stimulation of the GPCR signaling is vital to normal tissue homeostasis and depends on the duration of Gα subunit in the GTP-bound state. Studies have shown that hydrolysis of Gα-GTP to GDP inactivates the Gα subunit, permitting it to re-associate with the Gβ/Gγ dimer and recycled back to plasma membrane terminating all effector interactions. Additionally, novel feedback regulatory mechanisms by members of the GPCR kinase (GRK) family desensitizing agonist-bound GPCRs have been reported resulting in downregulation of chemokine signaling (Pitcher et al. 1998; Premont and Gainetdinov 2007; Robinson and Pitcher 2013).
4 Regulation of Expression of CXCR4
The entire transcription unit of the human CXCR4 gene has been characterized for elucidating the presence of potential binding sites for known transcription factors and its regulation. Based on the information available thus far, it is concluded that the local expression of chemokines and chemokine receptors in breast and various cancers are regulated at multiple levels, including transcription, translation, and protein degradation although no CXCR4 specific transcription factor has been reported till date.
Emerging evidence highlights the critical role of Hypoxia-inducible factor-1α (HIF-1α), a central mediator of tissue hypoxia, to induce upregulation in the expression of CXCR4 in MCF-7 and MDA-231 cells (Matteucci et al. 2007; Schioppa et al. 2003). Additionally, HIF-1α also induces SDF-1α expression on endothelial cells enabling their trafficking to distinctive niches of hypoxic regions; in doing so it stimulates recruitment of endothelial cells to the growing tumor along with their colocalization with tumor cells. It has been found that under normal oxygen tension, pVHL, the product of the von Hippel–Lindau tumor suppressor gene (VHL), induces degradation of HIF-1α. However, during hypoxia and circumstances harboring mutations in the VHL gene, HIF-1α degradation is compromised leading to its accumulation and stimulating expression of CXCR4. Notably, it has been shown that the promoter for CXCR4 retains a functional hypoxia response element attesting the regulation of CXCR4 expression by HIF transcription factor (Staller et al. 2003). Patients bearing renal clear cell carcinoma with inactivating mutations in the VHL gene express higher levels of CXCR4 than those without VHL mutations and associated with poor survival (Staller et al. 2003; Zagzag et al. 2005). Importantly, other transcription factors such as NF-kB and YY1 also influence the induction of expression of the chemokine receptor CXCR4 transcription. Nuclear factor-kappa B (NF-κB) is one of the key regulators of proper organogenesis of the mammary gland and plays an important role in the etiology of breast cancer. Constitutively, active NF-κB DNA-binding activity is detected in both mammary carcinoma cell lines and primary human breast cancer tissues and is responsible for overexpression of prometastatic, proangiogenic, and antiapoptotic genes in breast cancer (Badr et al. 2013a). Further CXCR4 expression in breast cancer cells is regulated by NF-κB, as well as by cytokines that induce NF-κB. A number of known activators of NF-κB, including TPA and CD30, have shown to induce CXCR4 (Caruz et al. 1998; Vinante et al. 2002) conveying the message that CXCR4 is one of the NF-κB target genes. Subsequent studies revealed a putative NF-κB binding site (5-GAGGCATTTCC-3, 230–240) within the −66 to +7 region of the CXCR4 promoter, which convey this important message that NF-κB directly regulates the expression of CXCR4 and directly involves in SDF-1α-mediated migration of breast cancer cells (Caruz et al. 1998; Helbig et al. 2003).
Furthermore, within proximal promoter region of CXCR4, consensus sequences complimenting Sp1 and nuclear receptor-1 (NRF-1) binding site have been identified (Moriuchi et al. 1997). It is worth noting NRF-1 binding sites are involved in transcriptional regulation of multiple mitochondrial genes; one may conceptualize that coordinated NRF-1 expression might contribute to cell’s increased metabolic demand in response to proliferative signals and cell migration. NRF-1 has been reported to upregulate CXCR4 expression in human rhabdomyosarcoma (Tarnowski et al. 2010), although it remains unknown in breast cancer since no studies have addressed this aspect.
5 Chemokines and Angiogenesis
Empirically, angiogenesis is the process of formation of new blood vessels towards tumor and constitutes an important feature for tumor cells to survive and facilitate growth. It is now widely recognized that breast and many other solid tumors require angiogenesis and fail to grow beyond a few millimeters in diameter in absence of angiogenesis. Vascular endothelial growth factor (VEGF)—a pro-angiogenic molecule—is a critical mediator of this process and potent endothelial mitogen that prompts a rapid and complete angiogenic response in normal and malignant tissues by sprouting new blood vessels. VEGF is secreted by a number of different cancer cell types and overexpressed not only by breast cancer cells but also by activated breast stromal cells suggesting an active role for the latter in tumor growth and angiogenesis (Folkman 1995). Interestingly, chemokines have been suggested to actively participate in the process of angiogenesis by inducing the recruitment and proliferation of endothelial cells to form vessel walls. Corollary to such observations, CXCR4/SDF-1α interaction leading to capillary tube formation of HUVECs in vitro and tumor angiogenesis in vivo has been recorded, and SDF-1α mediates angiogenic effects in part through the induction of VEGF and downregulation of negative regulator of VEGF such as, expression of the glycolytic enzyme phosphoglycerate kinase-1 (PGK1), an ATP-generating glycolytic enzyme that forms part of the glycolytic pathway and directly involved in CXCR4/SDF-1α signaling (Wang et al. 2007). Additionally, it has been shown that VEGF can induce the expression of CXCR4 on breast cancer cells as part of its autocrine function, thus coupling VEGF expression with the migratory potential of cells and to SDF-1α signaling (Bachelder et al. 2002). Moreover, it has been shown that mechanistically CXCR4/SDF-1α signaling cascade can persuade angiogenesis and progression of tumors by increasing expression of VEGF through the activation of PI3K/Akt pathway (Badr et al. 2013b).
6 Chemokine Receptors and Microenvironment
Researchers in molecular pathology over the past decade have shed light to understand the contribution of tissue microenvironment in cancer progression and conceded CXCR4-SDF1α signaling axis as the main driver regulating this interactive function. According to contemporary views, for invasion to proceed, it is important for tumor cells to lose cell-to-cell adhesion properties, reorganize their cytoskeleton, translate into epithelial-to-mesenchymal (EMT) phenotype, and modulate the surrounding extracellular matrix (ECM). To accomplish these physiological alterations, overexpression of proteolytic activities of matrix metalloproteinase (MMPs) assists in the degradation of ECM and basement membrane. In cell culture models, SDF-1α upregulated protein expression and increased the enzymatic activity of MMP-9, while SDF-1α inhibitors abolished this activity. These and other related studies confirm MMP-9 as SDF-1α responsive mediator instigating the degradation of ECM and assists in subsequent steps of cancer invasion and metastasis. Additionally, SDF-1α and CXCR4 expression dictate EMT phenotype characterized by the loss of epithelial markers (E-cadherin, Zeb-1) and gain of mesenchymal surface markers (N-cadherin, vimentin; Fig. 2). Yang and coworkers demonstrated that silencing of CXCR4 was associated with a decrease in the tumorigenic properties of MDA-MB-231 breast cancer cells, with reversion of EMT, and repression of MMP-9 along with reduction in the incidence of lung metastasis in mice (Yang et al. 2014). Furthermore, a permissive tumor microenvironment have been underscored for its vital role in fostering the growth of cancer cells in parallel with the existence of a dynamic reciprocal interaction between tumor cells and diverse variety of resident cells in stroma, including tissue-associated fibroblasts, inflammatory cells, endothelial cells, adipocytes, and mesenchymal stromal elements. In breast cancer, stromal fibroblasts often constitute a major fraction of the stromal cellular environment and express high levels of CXCL12, but not normal mammary fibroblasts (Allinen et al. 2004). Further, the significance of high-level expression of SDF-1α by carcinoma-associated fibroblasts (CAF) in promoting breast cancer progression has been well illustrated in a co-implantation tumor xenograft model. CAF isolated from surgically resected human breast carcinoma specimens promotes the growth of admixed breast carcinoma cells significantly more than do normal mammary fibroblasts derived from the same patients (Orimo et al. 2005). Of interest, it is only the breast fibroblasts but not skin fibroblasts that enhance the growth rate of primary breast carcinoma xenografts in vivo (Kang et al. 2005b). Further investigations revealed that CAFs, which exhibit the traits of myofibroblasts (as deduced from expression of α-smooth muscle actin- a characteristic marker of myofibroblasts), play a central role in promoting the growth of tumor cells through their ability to secrete SDF-1α and soluble IL-6 (Kang et al. 2005a) (Fig. 2). In addition to direct effects on breast cancer cells, CAFs also promote neo-angiogenesis by trafficking endothelial progenitor cells (EPCs) into tumor stromal microenvironment, an effect mediated again in part, by SDF-1α. Thus, SDF-1α secreted by CAF cells directly stimulates tumor growth interacting directly through the cognate receptor CXCR4 expressed by cancer cells, strengthening the notion that CAFs within invasive breast carcinomas contribute aggressively to tumor promotion in large part through the secretion of SDF-1α. These two major mechanisms by which fibroblast-derived SDF-1α promotes tumorigenesis have been confirmed in other tumor types as well. Another noteworthy feature involving interaction with tumor microenvironment relates to the adhesion receptors present on cell surface. Integrin ανβ6 is an important adhesion receptor that results in firm adhesion and transendothelial migration into tissues exhibiting chemokine gradients. Integrin ανβ6 has been found reportedly upregulated in SDF-1α/CXCR-induced cell migration.
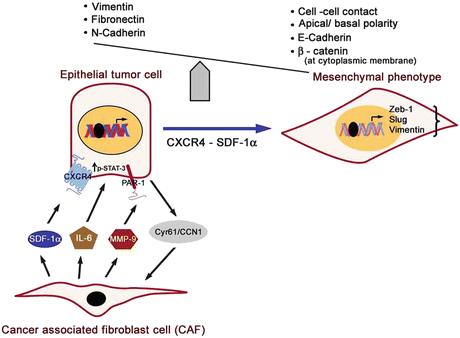

Fig. 2
Epithelial–mesenchymal transition (EMT) events and associated cellular and phenotype alterations and signaling interactions between cancer-associated fibroblasts (CAF) and tumor cells aiding growth and invasion of cancer cells. Cancer-associated fibroblasts secrete SDF-1α and IL-6 for paracrine actions on tumor cells activating CXCR4 and STAT-3 signaling. Matrix metalloproteinases (MMP-9) secreted by CAF cleave and activates protease-activated receptor-1 (PAR1) on the tumor cell surface stimulating growth and invasion pathways, while tumor cells secrete Cyr61/CCN1 stimulating MMP-1 production by adjacent fibroblasts creating a positive feedback loop potentiating tumor invasion
Mounting evidence suggests the bone microenvironment is critical in supporting the homing of the breast tumor cells into bone marrow similar to hematopoietic stem cells (HSC). Approximately, 65–75 % of patients with advanced breast cancer develop bone metastasis with shorter median survival times compared to those without bone metastasis (Kato et al. 2003). Soluble factors such as osteopontin (OPN) secreted by breast cancer cells mediate cell adhesion to bone matrix and initiate a complex network impairing osteoblast differentiation. Once the circulating tumor cells have embarked inside the bone marrow, the high concentration of SDF-1α therein downregulates CXCR4 expression which prevents these cells from migrating to sites expressing SDF-1α, highlighting specific regulation of CXCR4 expression within bone marrow microenvironment. Combined with SDF-1α-induced adhesion to bone stromal cells and growth factors (Connective tissue growth factor, TGFβ, Interleukin-11)-stimulated proliferation, results in the establishment and persistence of breast cancer bone metastasis. Collectively, the foregoing information provides enough support for the crucial role of microenvironment in maintenance of malignant behavior of tumor cells and consequent progression of the disease state.
Bioactive Natural Products
Naturally occurring chemokine antagonists hold prospects in era of drug discovery. Several natural chemopreventive compounds have been evaluated for attributes targeting CXCR4-SDF1α signaling axis leveraging a rationale-based generation of anti-metastatic protocol along with traditional chemo- and radio therapy. Several lines of evidence document anti-metastatic phenomenon of bioactive compounds in breast cancer along with pro-apoptotic activities in tumor cells and tissues. Among class of bioactive natural products, polyphenols constitute a large family of compounds with many component compounds being used to complement cancer therapies in cancer patients. We summarize below some natural plant and nonherbal agents capable of thwarting CXCR4-SDF1α signaling thus providing a window of opportunity to lower the risk of metastasis in women with higher risk and preventive approach through downregulation of this pathway in human. It has been reported that the breast cancer survivors are amongst highest users of dietary supplements some of which helps in delaying comorbid conditions associated with disease progression. Further elucidation of structure–activity relationship may prove useful in new drug design and development based on screening assays in preclinical models of breast diseases including cancer in general and breast cancer in particular.
Omega-3 Polyunsaturated Fatty Acids (n-3 PUFAs)
Ever since Endres et al. provided first evidence of optimal health beneficial actions of the key family of Omega-3 PUFA, the effect of n-3 PUFAs in prevention of chronic diseases has received considerable attention. n-3 PUFAs which include eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are biochemically classified as essential fatty acids that cannot be synthesized by mammals and, thus, their source of availability in human are basically through natural dietary sources such as fish, flax seeds, and nuts. In cell culture studies using MDA-MB 231 human breast cancer cell line, the effect of EPA and DHA on expression and activity of CXCR4 has been reported (Altenburg and Siddiqui 2009). n-3 PUFA treatment results in reduced surface expression of CXCR4 corresponding with reduced migration of treated cells. However, in vivo efficacy of n-3 PUFA in preclinical animal models of breast cancer needs to be evaluated before translation into clinical practice.
Tannic Acid
Tannins and tannic acid (polymer of gallic acid molecules and glucose) are water soluble polyphenols found widely distributed in plant kingdom including food grains and fruits. Although the mechanism is still being explored, tannins and tannic acid exert chemopreventive action as a result of inducing cellular death- apoptosis resulting in inhibition of tumor growth and antioxidative properties. Tannins and tannic acid have been identified as constituents of a herbal medicine Lianqiao (fruit of Forsythia suspensa), and under the laboratory culture conditions using MDA MB-231 cells, these molecules have been considered as a novel selective CXCL12/CXCR4 antagonist inhibiting the migration of CXCL12-induced migration of breast tumor cells, and prevents bovine aorta endothelial cell capillary tube formation (Chen et al. 2003).
Ginsenoside Rg3
This is the active natural triterpenoid saponin ingredient isolated from Panax ginseng. Using indirect investigational techniques, the influence of Rg3 on CXCR4 expression has been reported in cultured MDA-MB-231 breast cancer cell line (Chen et al. 2011). Based on Immunohistochemistry, chemotaxis, and wound healing mobility assays, it has been shown that Rg3 treatment at nontoxic dose range elicits a weak CXCR4 staining indicative of downregulation of the receptor, concomitant with diminutions in the number of migrated cells in CXCL12-elicited chemotaxis, and reduction in the width of the scar in wound healing assay attesting Rg3 efficacy with CXCR4 inhibition (Chen et al. 2011).
Baohuoside-I
This flavonoid constituent from Epimedium koreanum, an ingredient of Chinese traditional medicine, has the potential to suppress cancer metastasis. Baohuoside-I downregulates CXCR4 expression in a dose- and time-dependent manner in cervical cancer (HeLa) and breast cancer cells at mRNA and protein level, conforming to inhibition of CXCL12-induced invasion of both cervical and breast cancer cells (Kim and Park 2014). Thus, baohuoside-I may exert antimetastatic effect through the downregulation of CXCR4 expression.
Plumbagin
This is a bioactive naphthoquinone derived from the roots of plant Plumbago zeylanica with validatedanticancer activities documented against a wide variety of cancers. Plumbagin reportedly targets multiple cancer signaling proteins and pathways in breast cancer that plays an important role in cancer cell survival, proliferation, invasion, and metastasis of cancer cells and thus potentially associated with preventative and therapeutic values. Referencing breast cancer, plumbagin downregulates the expression of CXCR4 in breast cancer cells irrespective of their HER2 status (Manu et al. 2011). Further, in depth analysis reveals that plumbagin mediates the downregulation of CXCR4 at the transcriptional level along with inhibition of NF-κB activation. Additional information derived from extrapolative, functional proteomics-based tumor pathway platform confirms that NF-κB inhibition by plumbagin causes decrease in CXCR4 and other metastatic genes which coordinate well with the inhibition of CXCL12-induced migration and invasion of both breast and gastric cancer cells (Manu et al. 2011). This confirms plumbagin as novel blocker of CXCR4 expression and thus has the potential to suppress metastasis of cancer.
Zerumbone
This is a bioactive sesquiterpenoid derived from roots of subtropical Zingiber zerumbet. Zerumbone has been shown to downregulate chemokine receptor CXCR4 expression on HER2-overexpressing breast cancer cells in a dose- and time-dependent manner including downregulation of mRNA expression and inhibition of NF-κB activity; together these all lead to inhibition of CXCL12-induced invasion of breast tumor cells(Sung et al. 2008). Furthermore, an analogue of zerumbone, alpha-humulene, which lacks the carbonyl group, has been reported to be inactive in inducing CXCR4 downregulation confirming zerumbone as novel inhibitor of CXCR4 expression and has the potential to suppress cancer metastasis.
Celasterol
This compound is a pleiotropic natural ingredient of traditional Chinese medicinal plant—Tripterygium wilfordii. The antitumor activities of celasterol in different preclinical models of site specific cancers as well as, an anti-inflammatory and a novel HSP inhibitor have been documented. Celasterol can downregulate the CXCR4 expression on breast cancer MCF-7 cells stably transfected with HER2 oncogene which is known to induce the chemokine receptor (Yadav et al. 2010). Quantitative reverse transcription polymerase chain reaction and chromatin immunoprecipitation analysis reveal that downregulation of CXCR4 by celasterol is targeted both at transcription and translational levels, and that abrogation of chemokine receptor by celasterol leads to suppression of invasiveness of cancer cells induced by CXCL12, the ligand for CXCR4.
Xanthohumol
This is a polyphenol chalcone (2′, 4′,4-trihydroxy-6′-methoxy-3′-prenylchalcone) found in hops (Humulus lupulus) flower, and associated with multiple pharmacological activities including anticancer effects and suppresses CXCR4 expression in various cell types in dose and time responsive manner. The inhibitory effect of xanthohumol is not due to proteolytic degradation of the receptor, but initiated at the transcriptional level(Wang et al. 2012). At molecular level, xanthohumol blocks endogenous NF-κB activation, thus affecting the expression of CXCR4 in tumor cells. In line with this observation, zanthohumol could effectively abolish cell invasion induced by CXCL12 in breast cancer cells which one may anticipate a promising therapeutic agent in breast cancer treatment. More studies are warranted especially in in vivo model.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree