(1)
Department of Surgery, University of California, San Diego, 3855 Health Sciences Drive, La Jolla, CA 92093, USA
(2)
Department of Surgery, Memorial Sloan-Kettering Cancer Center, New York, NY, USA
Electronic supplementary material Supplementary material is available in the online version of this chapter at 10.1007/978-3-319-09342-0_25. Videos can also be accessed at http://www.springerimages.com/videos/978-3-319-09341-3.
Introduction
Utilization of minimally invasive techniques for gastric cancer surgery has increased in recent years. Laparoscopic distal gastrectomy for early-stage, distal gastric cancers is well established and is routinely practiced in the East where gastric cancer screening is routine. More than five randomized, prospective trials have confirmed improvements in short-term outcomes compared to open distal gastrectomy for patients with early-stage disease [1–6]. Laparoscopic resection of locally advanced and proximal gastric cancers, however, is not as well studied or widely performed. The two-dimensional view provided by the conventional laparoscope and limited range of motion of the instruments makes these complex resections challenging to perform laparoscopically. Controversy exists over the ability to perform an adequate lymphadenectomy laparoscopically in cases of locally advanced disease and over the safety of a laparoscopic esophagojejunal anastomosis in total gastrectomy.
The robotic surgery platform offers several technical advantages over laparoscopy. The camera provides a three-dimensional, magnified, high-definition view that is stable and is controlled by the primary surgeon. The articulated robotic instruments provide seven degrees of freedom and facilitate performance of difficult dissection and suturing. These advantages have led surgeons to investigate the use of the robotic platform for gastrectomy. Robot-assisted gastrectomy (RG) for gastric adenocarcinoma was first reported in 2003 [7, 8] and was first reported in the United States in 2007 [9]. Since that time, multiple retrospective series of RG for gastric adenocarcinoma have been published, almost all from the East [10–16]. The conclusions that can be drawn from these retrospective studies are limited due to great variability in inclusion criteria, surgeon experience, type of reconstruction performed, and the outcomes evaluated.
This chapter will describe the technical aspects of RG for gastric cancer and discuss considerations regarding the learning curve and patient selection. Additionally, the chapter will summarize current literature on RG for gastric cancer with a focus on outcomes and costs.
Technical Aspects of Robotic Gastrectomy (Video 25.1)
Patient Positioning and Port Placement
RAG is performed with the patient in the supine position on a split-leg table. The patients’ arms are tucked bilaterally with adequate padding of elbows and hands to avoid pressure points. The patient is secured to the table at the shoulders using foam blocks and heavy-duty adhesive tape applied circumferentially around the blocks and the table. Fixation is also applied at the hips with a safety belt and circumferentially at the knees. Footboards may also be applied at the feet as further means to avoid sliding during reverse Trendelenburg positioning. Once patient positioning is completed, it is important to place the patient in steep reverse Trendelenburg as a test to assure stability.
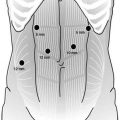
Port placement for RAG follows the same principles as for any robot-assisted procedure which include placement of the camera port at a distance of 15–20 cm from the target anatomy, placement of robot ports at least 8-cm apart from each other, and an assistant port at least 5 cm from adjacent robotic ports. While multiple variations of port placement have been described, the placement illustrated in Fig. 25.1 is recommended. Pneumoperitoneum is established with a Veress needle just off of the left costal margin. A 12-mm trocar is then placed in the midline above or below the umbilicus depending on the patient’s body habitus but with a goal of port placement 15–20 cm from the target anatomy. In the majority of cases, the infraumbilical position is best. Two additional 8-mm da Vinci ports are then placed on the left side, at least 8 cm from each other and slightly off-set from the plane of the camera port. An additional 12-mm port is placed in the right mid-clavicular line, and an 8-mm robotic port is placed within it. A 5-mm assistant port is placed further laterally on the right side, approximately at the anterior axillary line.
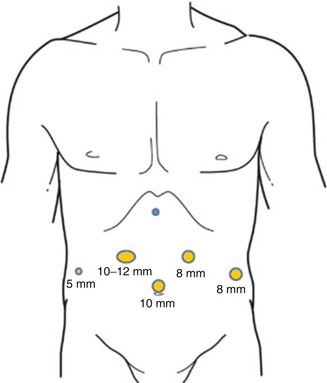

Fig. 25.1
Recommended port placement for robotic-assisted gastrectomy
At this point, the abdomen is explored for adhesions and for any evidence of peritoneal or extra-gastric disease. If a distal subtotal gastrectomy is to be performed and the lesion is not appreciable on the extraluminal surface, an endoscope is passed, and the lesion is localized. A silk stitch is placed laparoscopically to mark the level of transection of the stomach that will likely achieve a negative proximal margin. Once this is complete, the patient is placed in steep reverse Trendelenburg, and the robot is docked from directly over the patient’s head. Arms 1 and 3 are attached to the left-sided ports, and arm 2 is attached to the right-sided port within the large 12-mm port. A fenestrated bipolar grasper is placed in arm 2, and a harmonic scalpel or monopolar scissor is placed in arm 1. A grasping forcep, preferably a Cardiere, is placed in arm 3.
Procedural Steps
The procedure commences by flipping the greater omentum cephalad and locating the transverse colon. The omentum is carefully taken off of the colon proceeding in the direction of the splenic flexure. With careful dissection, the omentum is separated from the transverse mesocolon, and the lesser sac is entered. Visualization of the posterior wall of the stomach confirms entry into the lesser sac. The posterior wall of the stomach is then grasped by the bedside assistant and is retracted anteriorly and to the patient’s right side. The omentectomy is carried up toward the spleen and is stopped at the edge of the stomach just prior to reaching the short gastric vessels in a distal subtotal gastrectomy. For a total gastrectomy, the omentectomy is carried up to the esophageal hiatus, and the short gastric vessels are divided.
Once this is complete, the posterior wall of the stomach is grasped with the 3rd arm of the robot and is retracted toward the patient’s left shoulder. The omentectomy then proceeds toward the hepatic flexure of the colon and is completed. The omentum can be placed in the left upper quadrant on the anterior wall of the stomach at this point. The posterior attachments between the stomach and pancreas are then divided sharply or with the harmonic scalpel in the direction of the pylorus. The right gastroepiploic vessels are identified and dissected circumferentially at the level of the anterior border of the pancreas (Fig. 25.2). The vessels are divided at their origin with a vascular load of a stapler or with clips. If the stapler is to be used, arm 2 of the robot together with its associated 8-mm port is removed from the larger 12-mm port, and the stapler is passed by the bedside assistant.
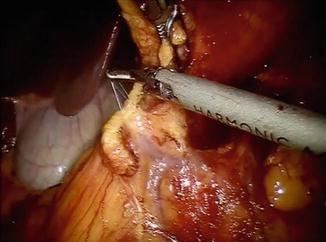

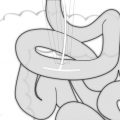
Fig. 25.2
Confluence of right gastroepiploic and right colic veins at anterior border of pancreas
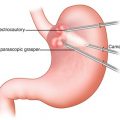
The pylorus is then identified by the vein of Mayo/white line, and attention is turned toward the suprapyloric region. The gastrohepatic omentum is incised with hook monopolar cautery or a harmonic scalpel in arm 1. The right gastric artery is identified and is ligated at its take-off from the proper hepatic artery with the harmonic scalpel. The lymphatic tissue along the hepatic proper and common hepatic artery is swept medially toward the specimen, and a window is created at the level of the pylorus. The posterior aspect of the pylorus and proximal duodenum is elevated off of the retroperitoneum with a combination of blunt dissection and use of the harmonic scalpel. A blue load of the stapler with bioabsorbable reinforcement is then introduced, and the proximal duodenum is stapled and divided just distal to the pylorus (Fig. 25.3).
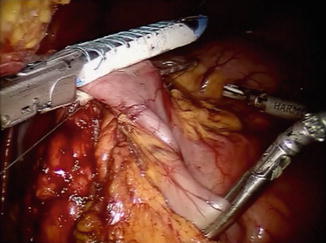

Fig. 25.3
Division of proximal duodenum just distal to pylorus
Once this is complete, the distal stomach can be retracted toward the patient’s left shoulder utilizing robot arm 3. The lymph node dissection that was started previously is then continued along the common hepatic artery toward the celiac axis and proximal splenic artery. The left gastric artery is identified at the celiac axis and is divided at its base with a vascular load of the stapler. The gastrohepatic omentum is further incised up to the level of the esophageal hiatus with the harmonic scalpel. For distal subtotal gastrectomy, the level 1 and 2 lymph nodes are peeled down off of the proximal stomach down to the level where the stomach will be divided. For a total gastrectomy, the distal esophagus is divided with stapler (blue load).
At this point, the specimen is placed in a specimen retrieval bag and is removed via the 12-mm port site in the right upper quadrant. The 12-mm port is then replaced, and the 8-mm robotic port attached to arm 2 is placed within it. Attention is then turned to the reconstruction.
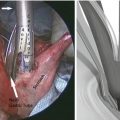
For a distal subtotal gastrectomy in which no more than half of the stomach was removed, we prefer an antecolic, Billroth II reconstruction is preferred. If greater than half of the stomach is removed or if a total gastrectomy is performed, we prefer a Roux-Y reconstruction is preferred. The colon is elevated cephalad, and the ligament of Treitz is identified. A mobile piece of jejunum approximately 30-cm downstream is selected and is used for the reconstruction. For a Billroth II or Roux-Y reconstruction to a gastric remnant, a side-to-side stapled gastrojejunostomy is created with a 60-mm laparoscopic stapler. The remaining enterotomy is sutured closed with a running 3.0 silk stitch with needle drivers in robot arms 1 and 2 (Fig. 25.4). For an esophagojejunostomy, an end-to-side anastomosis is created with a circular stapler. To facilitate this, the Orvil of the stapler is passed transorally on a nasogastric tube which is then pulled through the distal esophagus. The tubing is then gently detached from the Orvil and is removed through the 12-mm right upper quadrant port. The stapler itself is inserted into the Roux limb after removing the staple line with the Harmonic scalpel. Once the anastomosis is created, the open end of the Roux limb is closed with a linear stapler (Fig. 25.5). All staplers are inserted via the right upper quadrant 12-mm incision by the bedside assistant. For Roux reconstruction, a side-to-side stapled jejunojejunostomy is created approximately 70-cm downstream from the proximal anastomosis, and the remaining enterotomy is sutured closed. Mesenteric defects are also sutured closed in a running fashion with 3.0 Vicryl.
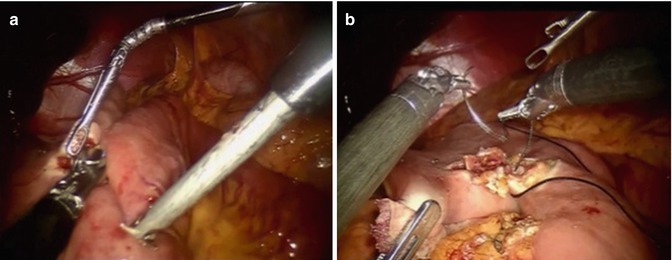
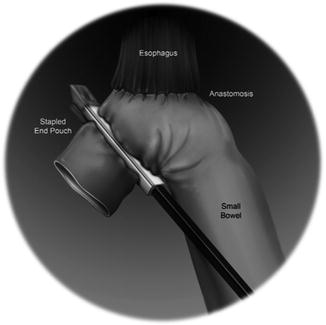

Fig. 25.4
(a) Creation of stapled side-to-side gastrojejunostomy. (b) Closure of gastroenterotomy

Fig. 25.5
Schematic diagram of stapled esophagojejunostomy following total gastrectomy
The Learning Curve
It is hypothesized that the learning curve for RG is less than that for LG due to the ergonomic and technical advantages provided by the robotic platform. There is evidence suggesting that this is the case for surgeons already experienced with advanced laparoscopy, but the recommended number of procedures required for learning varies. Some authors have suggested 20 cases for learning RG by advanced laparoscopic surgeons [11, 17]. More recently, Kim et al. performed a comprehensive, multidimensional analysis of the learning curve for laparoscopic versus robotic distal gastrectomy [14]. With their more rigorous statistical analysis of stability in operating time and “surgical success,” they found that 95 cases were required for learning RG, and 270 were required for LG. This was a retrospective analysis, however, and the surgeon had completed 177 LGs prior to the first robotic case. The number of robotic cases required might be greater for surgeons without prior laparoscopic experience.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree