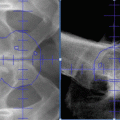
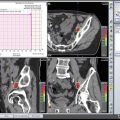
Fig. 12.1
Fascial coverings (curved arrow) over vasa deferentia (straight arrow) are released
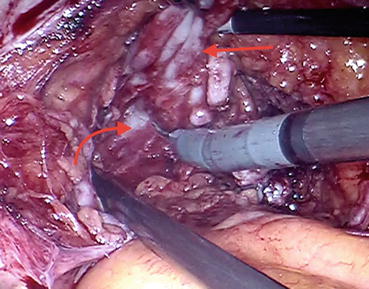
Fig. 12.2
Vas deferens and seminal vesicle being retracted (straight arrow) to develop the plane of dissection (curved arrow)
After completing the posterior dissection, the peritoneum is incised transversally through the medial umbilical ligament. The incision is extended on both sides in an inverted U fashion to develop the space of Retzius (Fig. 12.3). Then the endopelvic fascia is opened from the base of the prostate to the reflection of puboprostatic ligaments bilaterally using cold scissors (Fig. 12.4). After incising puboprostatic ligaments (Fig. 12.5), the levator fibers are pushed away from the prostate (Fig. 12.6) until the dorsal venous complex (DVC) and urethra are visualized which is necessary to get in a good and reliable DVC stitch.
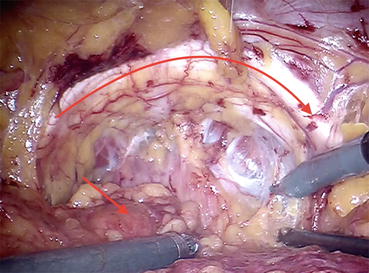
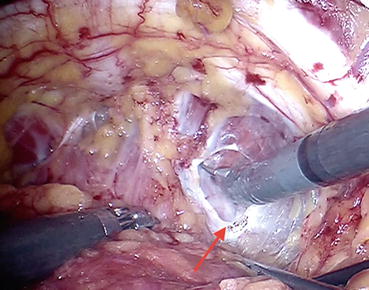
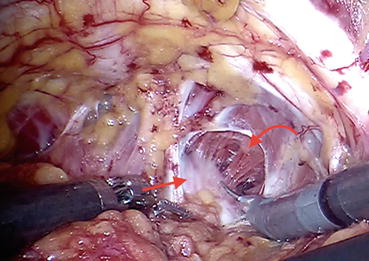
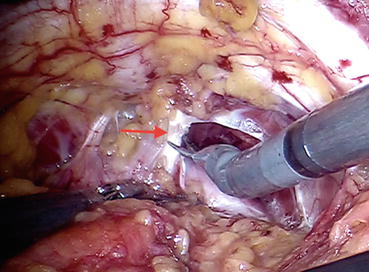

Fig. 12.3
Prevesical space (the space of Retzius or retropubic space), pubic arc (curved arrow), and bladder (straight arrow)

Fig. 12.4
Endopelvic fascia (arrow) incised with scissors

Fig. 12.5
Fibers of levator ani (curved arrow) are being pushed away from the prostatic surface (straight arrow)

Fig. 12.6
Puboprostatic ligament (arrow) is being incised by scissors
In order to ligate the DVC, robotic needle drivers are introduced, and the needle of an absorbable suture (zero polyglycolic acid) material is placed in the groove between the urethra and DVC (Fig. 12.7). The suture strength needs to be sufficient to allow the needle holders to pull up and perform a slip knot, which prevents the suture from loosening once it is tied (Fig. 12.8).
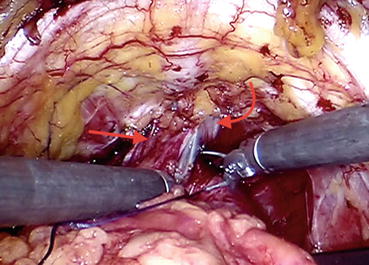
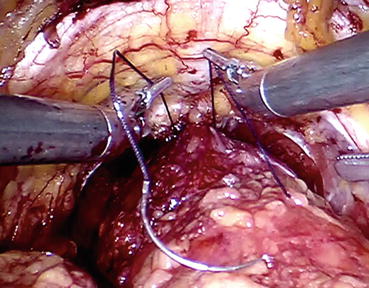

Fig. 12.7
Dorsal venous complex (DVC) stitch is being placed into the groove between the urethra (curved arrow) and DVC (straight arrow)

Fig. 12.8
Estimating the suture strength before applying the knot
After identifying the bladder neck via pulling on the urethral catheter and visualizing the balloon, the bladder is dissected off the prostate in the midline using a sweeping motion of the monopolar scissor. Once the anterior bladder neck is opened in the midline, bladder neck dissection begins on both sides. Afterward, the anterior urethra is divided, and the Foley catheter is retracted out of the bladder to apply upward traction (Fig. 12.9) which will be helpful while controlling the pedicle and exposing the posterior bladder neck.
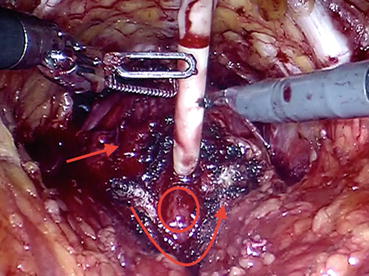

Fig. 12.9
The plane between the prostate (straight arrow) and anterior bladder neck (curved arrow) is developed, exposing the Foley catheter and the bladder mucosa (oval area)
Remaining peripheral bladder attachments are divided (Fig. 12.10) to flatten out the area of the posterior bladder neck , full thickness of which should be incised at the junction between the prostate and bladder. After grasping and applying traction to the lip of posterior bladder neck, the natural plane of dissection, which should be directed posteriorly and cranially, is appreciated more easily. This dissection should be carried out once the seminal vesicles are exposed.
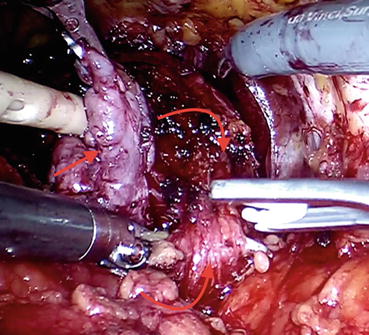

Fig. 12.10
Peripheral bladder attachments (curved arrow facing up) are being divided via clips. Note the dissection plane between the bulk of seminal vesicles (straight arrow) and prostate (curved arrow facing down) and bladder attachments
We usually opt for an interfascial nerve-sparing pedicle dissection in an antegrade manner during RARP, if it is oncologically feasible to do so. For this purpose, the periprostatic fascia is incised, and then the tissue on the lateral aspect of the prostate is gently spread in order to identify prostatic capsule and the neurovascular bundle (NVB) . No thermal energy is used during NVB dissection or ligation of the pedicle, and since the plane between the NVB sheath and the prostate capsule is relatively avascular, there should be minor bleeding once you are in the correct route. The NVB is then released in an antegrade fashion toward the apex (Fig. 12.11). The prostate pedicle can then be thinned out with sharp dissection, and this separation will allow safe placement of clip(s) on the pedicle away from the NVB.
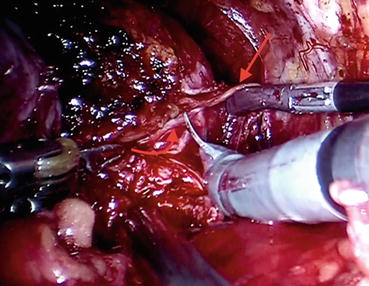

Fig. 12.11
Neurovascular bundle (NVB) (straight arrow) is released off the prostate capsule and prostatic fascia (curved arrow) via gentle sharp dissection
Cold scissors are used to divide the DVC and a long urethral stump is developed (Fig. 12.12). The urethra is then incised at the apex of the prostate to completely liberate the prostate (Fig. 12.13). In case of large prostate volume, large median lobe, or in patients with previous TURP, bladder neck reconstruction via application of transverse plication sutures can be necessary. Otherwise we usually attempt to preserve the bladder neck.
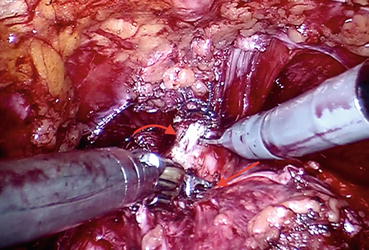
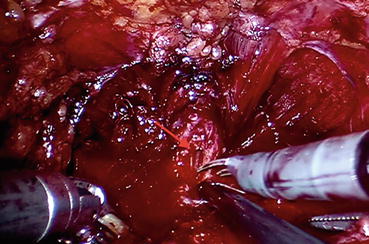

Fig. 12.12
Urethra (curved arrow) is being divided at the level of prostatic apex (straight arrow)

Fig. 12.13
Urethra is divided anteriorly; urethral lumen (arrow) becomes visible. Transection proceeds with the posterior aspect of the urethral circumference
After the specimen is extracted and the hemostatic measures are taken, the next step will be vesicourethral anastomosis. Before doing so, we usually apply the technique that has been proposed by Rocco et al. in 2006 [16], which includes restoration of the posterior aspect of the rhabdosphincter by reconstruction of the surrounding musculofascial plate in an attempt to fasten the recovery of continence post-RARP.
The urethra and bladder are reapproximated using running sutures as described by Van Velthoven [17]. Two 3/0 barbed sutures are used for this purpose, and first the posterior anastomosis is completed in a clockwise direction which is followed by the anterior anastomosis which is conducted in a counterclockwise fashion (Fig. 12.14). Then a Foley catheter (Chap. 22) is placed, and saline irrigation is done to confirm the watertightness of the anastomosis. Following this confirmation, a Jackson-Pratt drain is placed around the anastomosis, and all the trocars are removed under direct vision. Foley catheter is removed after 7 days without the routine need of a cystography.
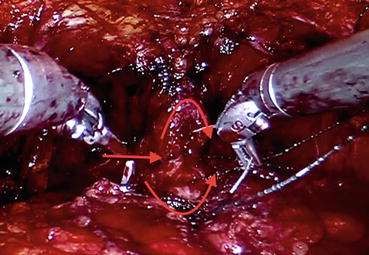

Fig. 12.14
Urethral stump (curved arrow facing down) is being anastomosed with the bladder neck (curved arrow facing up) via continuous sutures. Posterior lips of the two ends have been joined (straight arrow)
A total of 121 patients have undergone RARP in our clinic between May 2010 and August 2016. Mean patient age and mean pre-biopsy serum PSA level were 60.2 years (range, 40–76) and 6.6 ng/mL (range, 1–40), respectively. Mean ASA (American Society of Anesthesiologists) score and mean BMI (body mass index) of the patients were 1.6 (range, 1–3) and 28.2 kg/m2 (range, 22–37), respectively. Operations lasted 202.2 min (range, 115–440) on average, and mean estimated blood loss amount was 153.6 mL (range, 50–900). A total of four patients (3.3%) have received blood transfusions. Open conversion rate was 1.6% (n, 2). Sixteen complications that were of Clavien grade 2 and higher were recorded during the perioperative period of 11 patients. Distribution of the complications were as follows: grade 2 (n, 7 in seven patients), grade 3a (n, 2 in two patients), grade 3b (n, 6 in five patients), and grade 4 (n,1 in one patient). Mean duration of hospitalization was 4.5 days (range, 2–10). Overall positive surgical margin rate was 4.9% (n, 6). Distribution of the RP Gleason scores were as follows: 3 + 3 (3.9%), 3 + 4 (45.5%), 4 + 3 (11.8%), 4 + 4 (1.9%), 4 + 5 (3.9%), and 5 + 4 (2.9%). Mean tumor volume in the RP specimens was 2.89 cm3 (range, 0.2–18.5). After a mean follow-up duration of 13.8 months, biochemical recurrence was detected in a total of 12 patients (9.9%).
12.3 Perioperative Outcomes
Novara et al. has reported the perioperative outcomes and complications after robot-assisted radical prostatectomy in their systematic review and meta-analysis of the literature focusing on 110 papers published between January 1, 2008 and August 2011 [18]. Overall, mean operative time, mean blood loss, and mean transfusion rate were 152 min (range, 90–291), 166 mL (range, 69–534), and 2% (range, 0.5–5%), respectively. Mean duration of catheterization was 6.3 days (range, 5–8.6), and patients were hospitalized for a mean period of 1.9 days (range, 1–6). They additionally evaluated the outcomes in “difficult-to-treat” patient subpopulations. Higher BMI, higher prostate volume, prior BPH surgery, and the presence of median lobe were found to be associated with longer operative times. Larger prostates also lead to higher amount of blood loss, longer catheterization time, and longer length of hospitalization [18]. In the same study, the mean complication rate was calculated as 9% with the majority being of low grade (grades 1–3 according to the Martin criteria [19]), and the most prevalent complications were lymphocele /lymphorrhea (3.1%), urine leak (1.8%), and reoperation (1.6%).
Cumulative analyses of the studies comparing open RP versus robotic RP yielded that rates for blood loss and transfusion were in favor of RARP. On the other hand, operative duration and overall complication rates were similar between the two approaches. Considering the comparison between laparoscopic RP and robotic RP, transfusion rate, which was significantly lower in RARP, was the only parameter that showed a statistically significant difference. The authors denoted the impossibility of meta-analyses in terms of catheterization time and length of hospital stay [18].
Another systematic review and meta-analysis of the perioperative outcomes have revealed significantly less complications following RARP as compared to RRP and LRP [20]. Perioperative results of some of the most contemporary RARP series with available data are summarized in Table 12.1.
Table 12.1
Perioperative outcomes of selected RARP series
Study | N | Median/mean operative time, min | Median/mean blood loss, mL | Transfusion rate, % | Catheterization time, days | Length of hospitalization, days | Overall complication rate, % |
|---|---|---|---|---|---|---|---|
Menon et al. [21] | 30 | 288 | 329 | 7 | 11 | 1.5 | 6 |
Tewari et al. [22] | 200 | 160 | 153 | 0 | 7 | 1.2 | 3 |
Ahlering et al. [23] | 60 | 231 | 103 | 0 | 7 | 1 | 6.7 |
Rocco et al. [24] | 120 | 215 | 200 | NA | 6 | 3 | NA |
Krambeck et al. [25] | 286 | 236 | NA | 5.1 | NA | NA | 4.8 |
Rozet et al. [26] | 133 | 166 | 609 | 3 | 9.2 | 5.4 | 19.4 |
Drouin et al. [27] | 71 | 199 | 310 | NA | 8.1 | 4.4 | 8.4 |
12.4 Functional Outcomes
12.4.1 Erectile Function
Ficarra et al. have published a systematic review and meta-analysis of the studies that have reported outcomes related with erectile function in 2012 [28]. The 12- and 24- month potency rates ranged from 54 to 90% and 63 to 94%, respectively. In this paper, they have covered the RARP series that were published between 2008 and 2011 and included more than 100 patients. Age, baseline erectile function (International Index of Erectile Function (IIEF)-5 score), comorbidities (Charlson score), and extension of the nerve-sparing procedure represented the most important predictors of the risk of postoperative erectile dysfunction.
Furthermore, combination of these variables (age, IIEF score, and Charlson score) within the context of Briganti risk group stratification made it possible to provide a better overview to the patients about what to be expected after RARP in terms of erectile function recovery according to their individual risk category. Low-risk patients’ (age ≤ 60 year, baseline IIEF-6 > 21, and Charlson score ≤ 1) potency rates were 81.9% at 12 months, while the same rate was calculated to be 28.6% among high-risk individuals (age > 70, baseline IIEF-6 score ≤ 10, and Charlson score ≤ 2) [29].
Among the clinical series that included only the bilateral nerve-sparing procedures, 3-, 6-, 12-, and 24-month potency rates were 56%, 69% (50–86%), 74% (62–90%), and 82% (69–94%), respectively [28]. (A)thermal dissection of the neurovascular bundles [30], cold dissection of the cavernous nerve [31], countertraction during the nerve-sparing dissection [32], and the boundaries of the nerve-sparing dissection (intrafascial, interfascial, and extrafascial) represent the most common technical modifications that have been tested in terms of their potential beneficial role regarding postoperative erectile function recovery. Mean potency rates at 3, 6, and 12 months were 44, 50, and 66% (62–75%), respectively, in the clinical series using cautery (monopolar or bipolar) dissection and 52%, 78% (70–86%), and 81% (62–90%), respectively, in the studies using the athermal dissection [28]. Available data seem to support the use of cautery-free dissection or the use of pinpointed low-energy cauterization.
Despite the absence of high-quality level of evidence within this context, cumulative analyses showed better 12-month potency rates after RARP in comparison with RRP (odds ratio [OR], 2.84; 95% confidence interval [CI], 1.46–5.43; p = 0.002). On the other hand, the difference between RARP and LRP remained statistically insignificant (OR, 1.89; p = 0.21) [28].
In the recently published prospective randomized study comparing the outcomes of open versus robotic RP, which represent the relevant paper with the highest level of evidence so far, sexual function scores (sexual domain of EPIC and IIEF) did not differ significantly between the RRP group and the RARP group at 6 weeks postsurgery (30.70 vs. 32.70; p = 0.45) or 12 weeks postsurgery (35.00 vs. 38.90; p = 0.18) [33].
12.4.2 Continence
In their systematic review, Ficarra et al. have analyzed 51 articles that reported on urinary continence rates after RARP [34]. If urinary continence was defined more strictly as using no pads at all, the 12-month urinary incontinence rates ranged from 4 to 31%, with a mean value of 16%. However, if the definition was extended to involve a safety pad, then the incidence ranged from 8 to 11%, with a mean value of 9%. Age, body mass index, comorbidity index, lower urinary tract symptoms, and prostate volume were the most important preoperative parameters that influenced the risk of urinary incontinence after RARP. When different surgical approaches were compared in terms of continence recovery, RARP outperformed open (OR, 1.53; p = 0.03) and laparoscopic RP (OR, 2.39; p = 0.006) with a significantly better 12-month continence rate [34]. Approaching extra- or transperitoneally [35], bladder neck preservation [36], hypothermic nerve-sparing dissection using cold irrigation [31], dividing dorsal venous complex via athermal techniques initially and then selectively suture ligating prior to anastomosis [36], barbed versus monofilament sutures for urethrovesical anastomosis [37], and posterior/anterior reconstructions [38] represent the most commonly utilized/tested technical modifications that were employed in an effort to optimize continence recovery post-RARP. Available literature data seems in favor of total reconstruction with a significantly better continence outcome early postoperatively when compared with those who did not undergo such a reconstruction. However, it should be noted that beyond 6 months postoperatively continence rates of those who were reconstructed or not started to overlap.
A recent, prospective, controlled, nonrandomized, multi-institutional trial, comparing open and robotic approaches, showed that continence rates did not differ at 12 months postoperatively [39]. Yaxley et al. reported nonsignificant differences between the urinary function scores (urinary domain of EPIC) of open versus robotic RP at 6 (74.50 vs. 71.10; p = 0.09) and 12 weeks (83.80 vs. 82.50; p = 0.48) postoperatively in their randomized controlled phase 3 study [33]. Functional results of some of the most contemporary RARP series with available data are summarized in Tables 12.2 and 12.3.
Table 12.2
Continence outcomes of selected RARP series
Study | N | Assessment method | Criterion | 6 months, % | 12 months, % | 24 months, % |
|---|---|---|---|---|---|---|
Krambeck et al. [25] | 286 | NVQ | No leak | NA | 91.8 | NA |
Di Pierro et al. [40] | 75 | NVQ | No leak | NA | 89 | NA |
Geraerts et al. [41] | 64 | Physician reported | No leak | 90.3 | 89.8 | NA |
Rocco et al. [24] | 120 | Interview | No pad/one safety pad | 93 | 97 | NA |
Son et al. [42] | 146 | NVQ | No pad | 87.5 | 94.5 | 95.2 |
Joseph et al. [43] | 50 | Physician reported | No pad | 90 | NA | NA |
Table 12.3
Potency outcomes of selected RARP series
Study | N | Assessment method | Criterion | 3 months, % | 12 months, % | 24 months, % |
|---|---|---|---|---|---|---|
Krambeck et al. [25] | 286 | NVQ | Intercourse | NA | 70 | NA |
Di Pierro et al. [40] | 75 | NVQ | Presence of erection | 26
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|