Fig. 11.1
Open vs robotic PD trends between 2010 and 2015. Percentages of total PD completed via open (blue) and robotic (green) approach at the University of Pittsburgh Medical Center, Pittsburgh, PA, USA, between 2010 and 2015. The overall trend is towards increasing utilization of the robotic surgical platform for performance of PD procedures
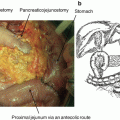
Our operative approach has previously been described [14, 20, 21] and utilizes the daVinci Robotic Surgical System (Intuitive Surgical, Sunny Valley, CA, USA). At the beginning of our experience, the S console system was utilized. Once the company upgraded to the Si, the computer interface and wrist capabilities of the robotic platform were better suited for this complex operation. We also have a Xi system and have performed robotic PDs on this system, as well; however, our preference remains use of the Si system. The procedure begins with laparoscopic evaluation. The configuration of port placements begins with placement of a 5 mm access port placed in the left subcostal region, utilizing an optical separator. This port will later be converted to a robotic 8 mm port. For malignant pathologies, once we confirm there is no metastatic disease, we place the remaining ports under direct visualization in the following fashion (Fig. 11.2): 12 mm camera port 2–3 cm above and to the right of the umbilicus in a patient with an average body habitus, and two additional 8 mm ports for the robotic arms in the right midclavicular line and the right anterior axillary line. A 12 mm port is placed in the left lower quadrant, which serves as a port for the assistant and later for specimen extraction, and a 5 mm port is also placed in the right lower quadrant for the assistant. These are situated between the camera port and its neighboring port on either side. Additionally, a self-retaining liver retractor is placed in the left upper quadrant. When placing these ports, care is taken to ensure that at least a hands-breath, or 5–6 cm, is between ports to allow for free movement of instruments. For the first 6 years, the beginning of the procedure was performed laparoscopically; however, in the past 2 years, we have converted to an almost entirely robotic approach, where we dock the robot after port placement. One key maneuver is to close the camera 12 mm port with a “figure of 8” stitch using a suture passer prior to docking the robot (Fig. 11.2).
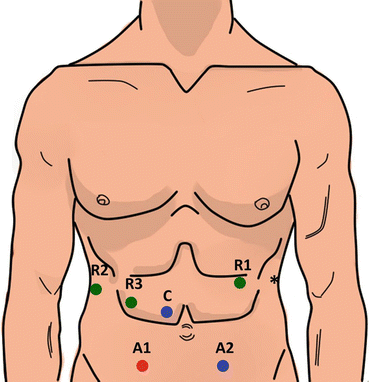

Fig. 11.2
Port placement configuration for robotic-assisted pancreaticoduodenectomy. Robotic 8 mm ports (R1, R2, R3) are used for the robotic arms. The 12 mm camera port (C) is placed above and to the right of the umbilicus. Assistant ports include a 5 mm port in the right lower quadrant (A1) and a 12 mm port in the left lower quadrant (A2), which then serves as specimen extraction site. The asterisk indicates a 5 mm self-retaining liver retractor
The robot is then docked directly over the head of the patient using the Si or at the patient’s right shoulder using the Xi. Our primary instruments for a majority of the resection include the hook monopolar in the right hand, the fenestrated bipolar in the left hand, and the cadiere or prograsp in the 3rd hand. The resection begins after entering the lesser sac through the gastrocolic omentum, followed by mobilization of the right colon then the duodenum by means of a Kocher maneuver. One trick to the operation is delivering the jejunum into the right upper quadrant by dissecting the ligament of Treitz until it is freed up about 40 cm, and then it is divided approximately 10 cm from the uncinate process. Then, the right gastric artery is taken with an energy device on the lesser curve, followed by the gastroepiploic artery along the greater curve. The stomach or proximal duodenum is transected. We favor a classic PD, but will occasionally perform a pylorus-preserving PD.
Once the stomach is divided, we move to the next step, which is dissection of the porta hepatis (Fig. 11.3). We start this dissection with removal of the hepatic artery lymph node. We think this is an important step for identification of the hepatic artery, portal vein (PV), and gastroduodenal artery (GDA). Once these structures are identified, we move lateral on the porta hepatis and identify the lateral aspect of the common bile duct (CBD), and then the lateral and posterior portal lymph nodes are dissected off the CBD and left attached to the specimen. Once this area is clear, we try to identify the PV and create a plane between it and the CBD. Then, we go back to the GDA and test clamp to make sure there is still adequate hepatic artery flow once clamped. If any question, we perform an ultrasound of the artery and test clamp under Doppler and ultrasound flow. We ligate the GDA with a vascular stapler and leave a clip on the staple line to mark the stump. Then, we dissect the CBD medially off the PV and once encircled, staple with a vascular load, as well. The benefit to dissecting laterally prior to stapling the GDA and CBD is to assure that there are no replaced or accessory hepatic vessels that need to be preserved (Fig. 11.3).
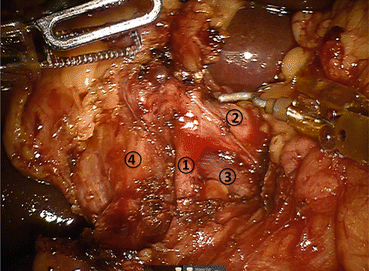

Fig. 11.3
Detailed view of portal dissection. The gastroduodenal artery (1) is isolated for ligation, typically via a vascular stapler and the stump is further reinforced with a clip. The common hepatic artery (2) and portal vein (3) can also be identified. The common bile duct (4) will also be transected using a stapler
Next, we dissect the inferior border of the pancreas, locate the SMV, and create a retro-pancreatic tunnel (Fig. 11.4). The pancreas is then divided with hot scissor electrocautery half way from anterior to posterior and inferior to superior. Then, care is taken to divide the pancreatic duct with “cold” scissor transection. Attention is then turned towards identifying the gastroepiploic and middle colic veins in relation to the SMV. The SMV is dissected fully to reveal the origin of these vessels prior to ligation. When possible, depending on the presence of a common trunk, the middle colic vein is preserved. The gastroepiploic vein is taken at its origin on the SMV. Once this is complete, we roll the SMV off the uncinate process and identify the first jejunal branches. We preserve these where possible; however, there are often numerous recurrent branches to the uncinate process requiring delicate dissection. Once the first jejunal is dissected off the uncinate, we identify the superior mesenteric artery (SMA). The magnified field of vision and articulating instruments of the robotic platform allow for both careful identification and management of the GDA and inferior and superior pancreaticoduodenal arteries, as well as smaller jejunal branches, which are often taken with an energy device. Where possible, a clip is placed on the staying side of the pancreaticoduodenals on the SMA. Furthermore, the visualization of the robotic system also allows for thorough resection of perivascular and peripancreatic tissue on the plane of Leriche (Fig. 11.5), allowing for thorough oncologic resection. The approach of the vascular groove and retroperitoneal margin varies based on gland texture and vascular involvement. A soft gland allows for a “back and forth” approach from anterior to posterior utilizing primarily an energy device. Anything with SMV involvement or a very firm gland may necessitate an “artery first” approach from inferior to posterior. If there is SMA involvement, we prefer a “hanging maneuver,” where the SMV is dissected above and below the first jejunal, which is then taken with a stapler or energy device. The SMA is then dissected under the SMV from medial to lateral. The advantages of the robotic approach assist in meticulous resection, but further aid in reconstruction (Figs. 11.4 and 11.5).
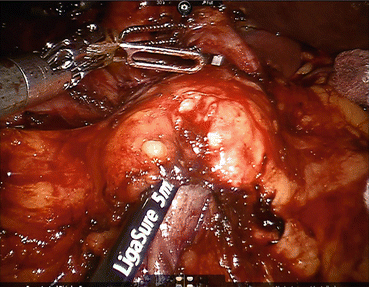
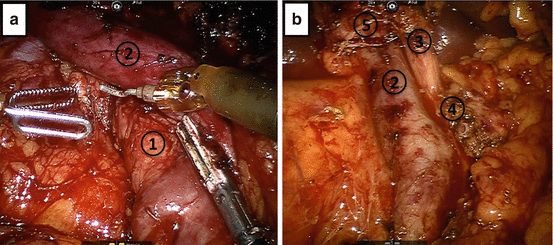

Fig. 11.4
Creation of the retro-pancreatic tunnel. Dissection proceeds along the inferior and superior borders of the pancreas, at the level of the pancreatic neck, and allows for creation of a tunnel beneath the pancreas and above the mesenteric vasculature

Fig. 11.5
Completed pancreaticoduodenectomy resection view. (a) The resection bed, with retraction of the superior mesenteric vein, shows careful dissection and removal of all the perivascular tissue along the plane of Leriche, clearing the superior mesenteric artery (1) and portal vein (2) margins. (b) After removal of the specimen, the view prior to reconstruction shows the dissected portal vein margin (2), the gastroduodenal artery stump (3), which is reinforced with a surgical clip, the cut edge of the pancreas (4), with a readily identifiable pancreatic duct, and the divided common bile duct (5)
The reconstructive process of PD is of utmost importance given the morbidity and mortality associated with anastomotic leakage and failure. We utilize a duct-to-mucosa fashion modified Blumgart pancreaticojejunostomy technique (Fig. 11.6) [22]. This two-layer anastomosis is typically constructed over a pancreatic duct stent (4, 5, or 7 French, Hobbs Medical, Stafford Springs, CT, USA). We use three 2-0 silk stitches on a V-20 needle for the outer layers and 5-0 monofilament sutures for the duct to mucosa stitches. We usually place 2 posterior and 2–5 anterior stitches depending on duct size. The hepaticojejunostomy or choledochojejustomy is then created, and bile duct texture and size are considered in determining the technique employed: a running technique is used with larger, thicker bile ducts and an interrupted technique is utilized with smaller, softer ducts (Fig. 11.7). Next, the gastrojejunostomy or duodenojejunostomy is created. For the past year, we have used a stapled technique where we sew the common enterotomy in two layers. Previously, we had performed a two-layer gastrojejunostomy or duodenojejunostomy. The advantages of the robotic surgical platform allow for complete minimally invasive reconstruction, as the magnified view allows for identification of even the smallest pancreatic duct, and the articulating instruments allow for dexterity and precision of suture placement. Following creation of the anastomoses, a 19 French round surgical drain is placed anterior to the pancreaticojejunostomy and the hepaticojejunostomy and posterior to the gastrojejunostomy. We use the falciform to create a pedicled tissue flap to cover the GDA stump. We hope this creates a tissue barrier to protect the artery from pancreatic secretions in the setting of a leak.
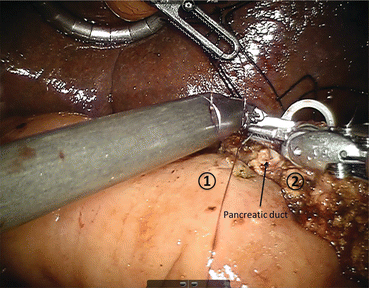
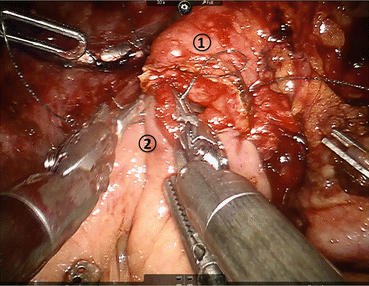

Fig. 11.6
Creation of pancreaticojejunostomy in modified Blumgart technique. The jejunum (1) is approximated to the pancreatic parenchyma (2) with 2-0 silk horizontal mattress sutures through the seromuscular layer of jejunum. Electrocautery is utilized to create a small enterotomy in the jejunum. Then, a duct-to-mucosa pancreaticojejunostomy is created using 5-0 PDS sutures over a Hobbs pancreatic stent (Hobbs Medical, Inc., Stafford Springs, CT, USA) to ensure duct patency. Finally, the anterior layer is created using 2-0 silk sutures to approximate the seromuscular layer of the jejunum to the pancreatic parenchyma

Fig. 11.7
Creation of the choledochojejunostomy. The common hepatic duct (1) is sutured to the jejunum (2) using interrupted absorbable 5-0 sutures for small ducts with or without a stent or running 4-0 V-LOC suture (Covidien, New Haven, CT, USA) for larger, thicker ducts (shown here)
Early Experience and Outcomes
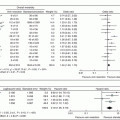
Early experience with robotic-assisted PD, as with laparoscopic PD, began slowly with small case series (Table 11.1). As previously discussed, Giulianotti performed and reported the first series of eight robotic PD in 2003. This robotic group had a longer mean operative time compared to open PD (490 vs 250 min), but roughly equivalent morbidity and length of stay (37.5% vs 32.1% and 20 vs 18 days, respectively) [15]. Continued experience by the same surgeon, at hospitals both in Italy and Chicago, IL, USA, was reported as a series of 50 robotic PD, 30 in Italy and 20 in the United States. In this series, mean operative time was 421 min with a conversion rate of 18.3%. Notably, a pancreatic fistula rate of 31.3% was reported. However, this elevated fistula rate includes patients who had sclerosis of the pancreatic duct performed in place of an anastomosis. The fistula rate in patients who had a pancreatic duct anastomosis performed was equivalent to reported rates for open procedures at 21% [23]. The first series of 24 robotic-assisted PD performed at this institution similarly had a postoperative pancreatic fistula rate of 21%, with 8% clinically significant fistula (International Study Group on Pancreatic Fistula grade B/C) and 29% Clavien-Dindo grade 3–5 complication rate [16]. Another series of 44 robotic PD at the University of Illinois at Chicago published in 2011 [24] showed decreased EBL (387 vs 827 ml), as well as increased lymph node retrieval (16.8 vs 11) compared to the open group. Overall, complication rates, including pancreatic fistula were similar between groups, as was the R0 resection rate. Notably, in this series, patients undergoing robotic PD were significantly older (63 vs 56 years), had higher ASA classifications (2.5 vs 2.15) and had higher BMI (27.7 vs 24.8). Despite this seemingly more complicated patient population, operative time in the robotic group was significantly shorter, with a mean of 444 min compared to a mean time of 559 min in the open PD group [24]. These first series of robotic PD showed overall trends towards prolonged operative times with equivalent rates of postoperative pancreatic fistula, but were encouraging enough to continue perfecting the approach.
Table 11.1
Outcomes of early robotic-assisted pancreaticoduodenectomy (PD)
Study | Time frame | Patients (n) | Operative time (min) | EBL (ml) | Lymph node (n) | R0 rate (%) | POPFa (%) | Morbidity (%) | LOS (days) | 30-Day mortality (%) |
|---|---|---|---|---|---|---|---|---|---|---|
Giulianotti et al. [15] | 10/00–11/02 | 8 | 490 | – | – | – | – | 37.5 | 20 | 12.5 |
Giulianotti et al. [23] | 10/00–01/09 | 50 – 30 Italy – 20 USA | 421 – 312 (Italy) – 351 (USA) | 394 – 261 (Italy) – 323 (USA) | – 21 (Italy) – 14 (USA) | – 100 (Italy) – 79 (USA) | 31.3 – 21 (PJ) | – | 22 – 28.7 (Italy) – 12.5 (USA) | 1.5 |
Buchs et al. [24] | 01/02–05/10 | 44 | 444 | 387 | 16.8 | 90.9 | 18.2 | 36.4 | 13 | 4.5 |
Zureikat et al. [16] | 10/08–02/10 | 24 | 512 | 320 | – | – | 21 | – 33 Clavien 1–2 – 25 Clavien 3–4 | 9 | 4.2 |
Boggi et al. [25] | 10/08–12/11 | 34 | 597 | 220 | 32 | 100 | 38.2 | 55.8 – 41.2 Clavien 1–2 – 14.7 Clavien 3–4 | 23 | 0 |
Zhou et al. [26] | 01/09–12/09 | 8 | 718 | 153 | – | 100 | 50 | 25 | 16.4 | 0 |
Chalikonda et al. [27]
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|