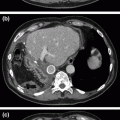
Fig. 25.1
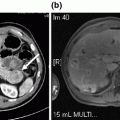
Preoperative imaging demonstrating resectable pancreatic head mass. a Arterial phase shows hypoenhancing mass in the pancreatic head (arrow). b Portal venous phase demonstrates fat plane (arrow) between mass and SMV at the level of the splenic vein
Following normalization of his serum bilirubin, tumor markers were significant for a cancer antigen 19-9 (CA19-9) of 149 U/ml (normal < 37 U/ml). Based on criteria from the NCCN, SSO, and AHPBA [11–13], the patient was classified as having resectable pancreatic cancer. The patient was discussed at multidisciplinary tumor board. Curative intent treatment options offered to this patient included preoperative chemotherapy on or off protocol versus surgery upfront followed by adjuvant chemotherapy. Our patient chose to enroll in UPCI protocol 13-074 (two cycles of gemcitabine/nab-paclitaxel with or without the autophagy inhibitor hydroxychloroquine). He completed therapy and underwent repeat staging contrast-enhanced CT of the chest, abdomen, and pelvis, which demonstrated no distant disease and a stable primary. He had a favorable biochemical response, with CA19-9 decreasing to 28.1 [10]. Therefore, decision was made to proceed with robotic-assisted pancreaticoduodenectomy (PD).
Technical Pearls of Robotic-Assisted Pancreaticoduodenectomy
Entry into lesser sac and mobilization of right colon
Kocher maneuver and opening of ligament of Treitz
Jejunal and gastric transection
Portal dissection
Remove common hepatic artery node
Identify and ligate GDA
Identify suprapancreatic portal vein and begin tunnel
Portal lymphadenectomy and transection of bile duct
Infrapancreatic SMV dissection and completion of the retropancreatic tunnel
Division of the pancreas
Uncinate dissection and specimen retrieval
Cholecystectomy (if gallbladder present)
Reconstruction.
Management
Pancreatic cancer is fundamentally a systemic disease in a majority of the patients at the time of diagnosis. This requires the clinician to integrate the timing of local control with the systemic management of this disease. The traditional sequencing of surgery followed by chemotherapy has proven inadequate despite dramatic improvements in mortality following the PD over the last 30 years, and morbidity of the open operation remains high. This high morbidity following the traditional open PD limits receipt of critical systemic chemotherapy that is required to improve survival. Several groups have demonstrated that postoperative complications affect ability to receive critical systemic chemotherapy with nearly 48% of subjects in one study never progressing to adjuvant therapy [4, 14]. There are several approaches to circumvent this clinical dilemma. First, it has been demonstrated that reversing the sequencing of surgery and chemotherapy results in more subjects receiving both modalities [15]. Second, we can reduce the morbidity of the surgery through the use of minimally invasive surgical (MIS) approaches. At our institution, we follow an innovative and aggressive algorithm in a majority of patients that favors administration of newer, more effective systemic chemotherapies preoperatively, followed by MIS PD and subsequent adjuvant chemotherapy. One additional potential benefit to this approach is that we are able to tailor the adjuvant chemotherapy based on the histopathologic response to the preoperative regimen.
Robotic Pancreaticoduodenectomy
Our institution utilizes seven ports for robotic-assisted pancreaticoduodenectomy, including a 10-mm camera port, three robotic arms, a 5-mm liver retractor, and two assistant ports. Port placement configuration is depicted in Fig. 25.2. Following abdominal access and insufflation, the abdomen is inspected for metastatic disease. Provided none is found, we proceed with mobilization, the first few steps of which can be performed laparoscopically or with robotic assistance. The patient is positioned in steep reverse Trendelenburg. We first enter the lesser sac through the gastrocolic ligament and continue dissection laterally to fully mobilize the right colon, exposing the duodenum. Next, a Kocher maneuver is performed to the level of the left renal vein, taking all the retroperitoneal attachments off of the pancreas and exposing the medial and inferior borders of the superior mesenteric artery (SMA). The ligament of Treitz is opened, allowing the proximal jejunum to be pulled underneath the SMA. The jejunum is transected with an endovascular stapler, and the mesentery is divided with a bipolar vessel sealer (LigaSure) at the border of the mesentery and serosa, completing the Kocher maneuver and linearizing the duodenum. We then divide the greater omentum at the level of the gastric antrum, and the right gastroepiploic artery is taken with the LigaSure. The lesser omentum is opened and the stomach is transected with an endovascular stapler. One unique aspect of the robotic PD is the inability of the surgeon to palpate the plain between the uncinate and SMA, as is classically described in the open approach. The decision of resectability and need for vascular resection must be appreciated before the operation based on adequate compute tomography. Thus the sequencing of the robotic PD is slightly different, and the jejeunum and stomach are divided early.
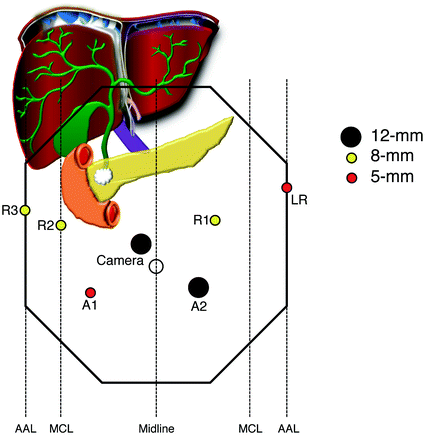

Fig. 25.2
Standard port placement for robotic-assisted pancreaticoduodenectomy. The peritoneum is accessed with a 5 mm optical viewing trochar using a 0° laparoscope in the left upper quadrant. This port is later exchanged for an 8 mm robotic port for Arm 1. The camera should be placed approximately 2 cm to the right and 2 cm above the umbilicus, to align with the SMV and optimize visualization of the uncinate dissection. Port placement can be moved up or down depending on the distance from the xiphoid process to the umbilicus (up for longer distance, down for shorter distance). The assistant ports should be placed approximately halfway between the camera and R2 (RLQ) and R1 (LLQ), respectively. The LLQ port site serves as the extraction site, and is converted to a GelPort following removal of the specimen
At this point, the robot is docked, if not already done. With the operating surgeon at the robotic console and the assisting surgeon positioned at the bedside between the patient’s legs, the portal structures and retropancreatic tunnel are dissected. The common hepatic artery (CHA) is exposed by identification and removal of the CHA lymph node. CHA exposure is continued until the right gastric and gastroduodenal arteries (GDA) are exposed (Fig. 25.3a). CHA flow is confirmed on ultrasound by Doppler and color flow after vessel-loop facilitated occlusion of the GDA prior to GDA ligation. The suprapancreatic portal vein is identified at the apex of the triangle formed by the common hepatic artery, GDA, and superior border of the pancreas. The avascular plane is developed in a cephalad-to-caudad direction, thereby beginning the retropancreatic tunnel from above. We then identify the common bile duct (CBD), and all lymphatic tissue lateral and posterior to it is cleared inferiorly toward the specimen. An aberrant or replaced right hepatic artery, if present, will be identified posterior to the CBD, and should be dissected circumferentially and traced proximally toward the SMA. The CBD is transected with an endovascular stapler, as we have found that this minimizes bile spillage that is difficult to evacuate during the uncinate dissection (alternatively a bulldog clamp can be placed.) The peritoneum overlying the inferior border of the pancreas is opened, and dissection is carried down until the infrapancreatic SMV is identified. The retropancreatic tunnel is completed (Fig. 25.3b) and the pancreas is transected with electrocautery; “cold” transection is reserved for the duct.
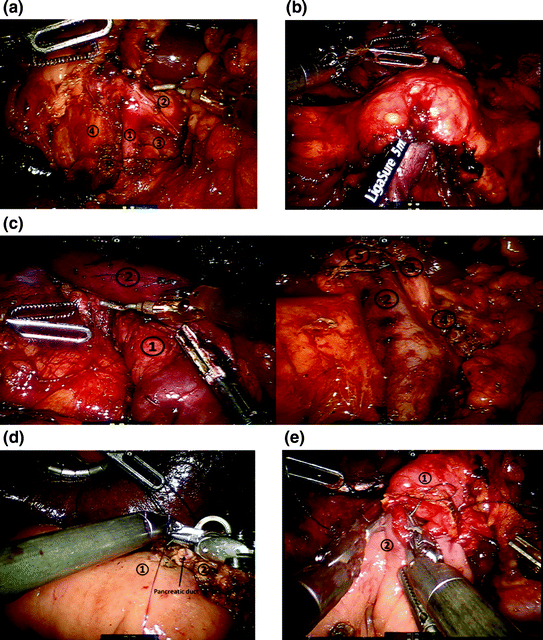

Fig. 25.3
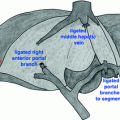
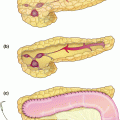
Robotic-assisted pancreaticoduodenectomy. a Detailed view of portal dissection. The gastroduodenal artery (1) is isolated for ligation, typically via a vascular stapler and the stump is further reinforced with a clip. The common hepatic artery (2) and portal vein (3) can also be identified. The common bile duct (4) will also be transected using a stapler. b Creation of the retropancreatic tunnel. Dissection proceeds along the inferior and superior borders of the pancreas, at the level of the pancreatic neck, and allows for creation of a tunnel beneath the pancreas and above the mesenteric vasculature. c Completed pancreaticoduodenectomy resection view. Left panel with retraction of the superior mesenteric vein, shows careful dissection and removal of all the perivascular tissue along the plane of Leriche, clearing the superior mesenteric artery (1) and portal vein (1) margins. Right panel shows the dissected portal vein margin (2), the gastroduodenal artery stump (3), which is reinforced with a surgical clip, the cut edge of the pancreas (4), with a readily identifiable pancreatic duct, and the divided common bile duct (5). d Creation of pancreaticojejunostomy in modified Blumgart technique. The jejunum (1) is approximated to the pancreatic parenchyma (2) with 2-0 silk horizontal mattress sutures through the seromuscular layer of jejunum. Electrocautery is utilized to create a small enterotomy in the jejunum. Then, a duct-to-mucosa pancreaticojejunostomy is created using 5-0 PDS sutures over a Hobbs pancreatic stent (Hobbs Medical, Inc., Stafford Springs, CT, USA) to ensure duct patency. Finally, the anterior layer is created using 2-0 silk sutures to approximate the seromuscular layer of the jejunum to the pancreatic parenchyma. e Creation of the choledochojejunostomy. The common hepatic duct (1) is sutured to the jejunum (2) using interrupted absorbable 5-0 sutures for small ducts with or without a stent, or running 4-0 V-LOC suture (Covidien, New Haven, CT, USA) for larger, thicker ducts (shown here)
After the pancreas is divided, attention is turned to dissecting the retroperitoneal margin and uncinate process. Special attention is given to the recurrent uncinated arterial and venous branches off of the SMA and SMV, as they are easily evulsed and can be a source of major intraoperative blood loss. Cephalad, we identify individually ligate the superior pancreaticoduodenal vein. The final resection bed is depicted in Fig. 25.3c. If the gallbladder is in situ, a cholecystectomy is performed at this point. The specimen is retrieved through the left lower quadrant assistant port, which must be enlarged. Pneumoperitoneum is reestablished with placement of a GelPort.
The enteric reconstruction is then carried out with meticulous attention, as most morbidity and mortality of PD is attributed to anastomotic leakage and failure. First a duct-to-mucosa modified-Blumgart pancreaticojejunostomy technique is performed (Fig. 25.3d), typically over a pancreatic duct stent [16]. We typically use 3 2-0 silk for the transpancreatic/seromuscular sutures, with the middle suture straddling the pancreatic duct. Once tied in place, an enterotomy is created, and we use 5-0 polydiaxanone for the duct-to-mucosa anastomosis. The anterior seromuscular layer is then placed. The bilio-enteric anastomosis is constructed, either by interrupted or continuous suture technique, depending on duct size; a running technique is employed for larger, thicker bile ducts, and an interrupted technique is used for smaller, softer ducts (Fig. 25.3e). Third, the gastrojejunostomy is performed by a stapled technique and sutured closure of the common enterotomy is done in two layers. Alternatively a “handsewn” gastrojejunostomy (or duodenojejunostomy) in a two-layered fashion can be formed. Following creation of the anastomoses, a 19 French closed suction, round, fluted surgical drain is placed anterior to the pancreaticojejunostomy and the hepaticojejunostomy, but posterior to the gastrojejunostomy. The falciform ligament is used to create a pedicled tissue flap to cover the GDA stump, which is marked with a 10 mm clip in case postoperative angiography is needed [17]. Unanticipated vascular involvement of the superior mesenteric vein or portal vein can be resected for an R0 resection due to the precision, control, and dexterity afforded by the robotic platform. We do not typically leave a nasogastric tube, as there is no evidence to support its routine use.
Postoperative care is focused on early diet advancement as tolerated, and conservative intravenous fluid management, titrated to hemodynamic parameters, and urine output through a modified enhanced recovery after surgery (ERAS) pathway. The ICU is not routinely used. We use a modified Verona protocol [18] to manage the operative drain. Serum and drain amylase are measured on POD1 and POD3. If POD 1 amylase is less than 5000 and decreases by POD 3 in a clinically stable patient, the drain is removed on POD3.
Following robotic-assisted pancreaticoduodenectomy, our patient was discharged home on postoperative day 5 after an uneventful recovery. Final pathology demonstrated a poorly differentiated adenocarcinoma with Evans Grade IIB treatment [19] effect, positive for perineural invasion without definitive lymphovascular invasion, 0/65 lymph nodes positive for metastases, and negative (R0) resection margins.
Alternative Approaches and Controversies
Open pancreaticoduodenectomy
Higher morbidity
Fully laparoscopic pancreaticoduodenectomy
Steeper learning curve
Less easily disseminated.
Perioperative Outcomes Following Robotic PD
In 2006, one of the largest series of open PD to date was reported by Johns Hopkins, with 1,432 cases for pancreatic malignancies. Winter et al. reported a mean operative time of 380 min, a mean blood loss of 800 mL, mean length of stay of 9 days, 58% R0 resections, 5% pancreatic fistula rate (pre-ISPFG criteria), and a 2% mortality rate [20]. This impressive study provides a metric for comparison of outcomes when describing a new technical platform such as robotic-assisted PD.
Similar to the adoption of laparoscopic PD, early reports of robotic PD began with small case series; Giulianotti performed and reported the first series in 2003 [21]. Over time, experiences grew, leading to larger series. Data suggested similar oncologic outcomes, decreased blood loss, increased operative time, and increased cost (Table 25.1). A recent review of studies published before 2012 evaluated five series of robotic PD, including 131 patients; the (weighted) mean operative time was 510 min and complications occurred in 38.9% of patients, including 26% postoperative pancreatic fistula and 2.3% mortality [22].
Table 25.1
Peri-operative outcomes of robotic-assisted pancreaticoduodenectomy
Author | Year published | Patients (n) | Time (min) | EBL (mL) | R0 resection (%) | Lymph nodes harvested (n) | POPF(%) | LOS (days) | 30-day mortality (%) |
|---|---|---|---|---|---|---|---|---|---|
Guilianotti [29] | 2010 | 50 | 568
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|