Advantages of robotic surgery
Disadvantages of robotic surgery
3D binocular vision
Loss of haptic feedback
Increased dexterity
Expensive, high maintenance costs
7 degrees of freedom
High start-up costs
Near 540-degree motion
Increased staff requirements
Elimination of tremor
Limited change of patient position
20–30× magnification
Cumbersome
Ability to scale motions
Relatively new technology
Ability to perform micro-anastomoses
Difficulty operating in multiple abdominal quadrants
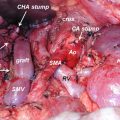
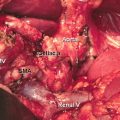
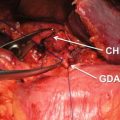
27.5 Robotic Pancreaticoduodenectomy (PD)
In one of the first large series on robotic PD, Giulianotti et al. reported on 134 robotic pancreatic resections, including 50 robotic PDs [17]. An initial series of 132 RPDs at the University of Pittsburgh reported 30- and 90-day mortality of 1.5% and 3.8%, respectively. Grade 3–4 complications (according to the Clavien-Dindo classification system [18]) were reported at 10% and 11%, respectively. These findings are in line with additional smaller studies, which have demonstrated postoperative morbidity and complication rates similar to open PD [19–21]. Rates of pancreatic fistula seen in these robotic series compare favorably to many of the largest series of patients undergoing open PD [22, 23]. In a very large series from the Johns Hopkins Medical Center, the authors reported a mean operative time of 380 min for the procedure, a mean blood loss of 800 mL, 58% R0 resection, and a mean length of stay of 9 days [24]. As can be seen from the previously noted robotic series and as noted in Table 27.2, outcomes approach equivalence with increasing proficiency. Additionally, oncologic outcomes appear to be similar in both open and robotic PD, with one series demonstrating an advantage in lymph node yield with the robotic approach [19].
Table 27.2
Comparison of outcomes of robotic pancreaticoduodenectomy
Series | Year | Patients (n) | Malignancy (%) | Time (min) | EBL (ml) | R0 resection (%) | Lymph nodes (n) | Length of stay (days) | 30-day mortality (%) | Fistula (%) | Conversions (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
Narula [25] | 2010 | 5 | 20 | 420 | NR | 100 | 16 | 9.6 | NR | NR | 37.5 |
Giulianotti [17] | 2010 | 50 | 100 | 568 | 394 | 90 | 18 | 22 | 8 | 38 | 22 |
Buchs [19] | 2011 | 44 | 75 | 444 | 387 | 90.9 | 16.8 | 13 | 4.5 | 18 | 4.5 |
Zhou [21] | 2011 | 8 | 100 | 718 | 153 | 87.5 | NR | 18.4 | NR | 25 | NR |
Lai [20] | 2012 | 20 | 75 | 491.5 | 247 | 73.3 | 10 | 13.7 | 0 | 35 | 5 |
Chalikonda [26] | 2012 | 30 | 46.7 | 476.2 | 485.8 | 100 | 13.2 | 9.79 | 3 | 7 | 10 |
Boggi [27] | 2013 | 34 | 64.7 | 597 | 220 | 100 | 32 | NR | 0 | 38.2 | 0 |
Zureikat [28] | 2013 | 132 | 80.3 | 527 | 300 | 87.7 | 19 | 10 | 1.5 | 17 | 8 |
Bao [29] | 2014 | 28 | 100 | 431 | 100 | 63 | 15 | 7.4 | 7 | 29 | 14 |
Having established safety and feasibility of RPD, the Pittsburgh authors sought to identify its learning curve. In a series of 200 consecutive RPDs, the learning curve was found to be 80 cases for operative time (581–417 min), 40 cases for fistula rate (27–14 %), and 20 cases for minimizing blood loss and conversion (600–250 ml and 35–3%, respectively) (all P < 0.05) [30].
27.6 Robotic Distal Pancreatectomy (DP)
In comparison to laparoscopic and robotic PD, minimally invasive DP has enjoyed a much broader acceptance, allowing for comparisons between the approaches. In a recent retrospective series of 35 patients comparing robotic and laparoscopic DP, operative time was found to be significantly longer in the robotic approach with no significant difference in overall morbidity rate [31]. However, there were nonsignificant trends favoring the robotic approach in operative blood loss and postoperative stay. A second series by Butturini et al. of 43 patients (21 robotic) confirmed the equivalence of robotic DP in regard to these end points, as well as no significant difference in lymph node yield [32]. In a series from the University of Pittsburgh, a significant decrease in conversion rate to open was noted with robotic DP compared to laparoscopic DP [33]. In this study, which minimized selection bias through propensity matching, the R0 resection rate and lymph node yield favored the robotic approach, while the laparoscopic group had a greater conversion rate despite having less pancreatic cancers compared to the robotic cohort.
Similar to the data on RPD, the Pittsburgh group sought to identify the learning curve associated with RDP. In analyzing the first 100 RDPs, the conversion rates were only 2%, despite a significant proportion (30%) of resections being for pancreatic ductal adenocarcinoma [34]. Significant reductions in operative time were observed after 20 cases, with optimization of performance noted after 40 cases. In a series of their initial 55 robotic DPs, Napoli et al. demonstrated low rates of serious morbidity, no conversions to laparotomy, and a learning curve similar to the experience at the University of Pittsburgh [35]. Additionally, series have also reported improved rates of splenic preservation utilizing the robotic approach [36, 37] (Table 27.3). These findings would seem to support robotic DP as a safe, oncologically sound procedure with a relatively short learning curve.
Table 27.3
Comparison of outcomes of robotic distal pancreatectomy
Series | Year | Patients (n) | Time (min) | EBL (ml) | Fistula (%) | Conversions (%) | Spleen preservation (%) | Lymph nodes (n) | LOS (days) | R0 resection (%) | 30-day mortality (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
Waters [38] | 2010 | 17 | 298 | 279 | 0 | 2 | 65 | 5 | 4 | 100 | 0 |
Giulianotti [17] | 2010 | 46 | NR | NR | 9 | 3 | 50 | NR | NR | NR | 0 |
Kang [37] | 2011 | 20 | 348 | 372 | NR | NR | 95 | NR | 7 | 95 | 0 |
Suman [39] | 2013 | 49 | 203 | 100 | 20 | 18.4 | 30 | 4.5 | 5 | 92.5 | 0 |
Hwang [40] | 2013 | 22 | 398 | 361.3 | 9.1 | 0 | 95.5 | NR | 7 | 100 | 0 |
Zureikat [28] | 2013 | 83 | 256 | 150 | 43 | 2 | NR | 16 | 6 | 97 | 0 |
Daouadi [33] | 2013 | 30 | 293 | 212 | 13 | 0 | 7 | 18.6 | 6 | 100 | 0 |
Chen [36] | 2015 | 69 | 150 | 100 | 24.6 | 0 | 95.7a | 15.5 | 11.6 | 100 | 0 |
Lai [31] | 2015 | 17 | 221.4 | 100.3 | 41.2 | NR | 52.9 | NR | 11.4 | NR | 0 |
Lee [12] | 2015 | 37 | 213 | 193 | 8 | 38 | 8 | 12 | 5 | 100 | 0 |
27.7 Robotic Central Pancreatectomy (CP)
In comparison to DP, CP is less frequently described in the literature. This is potentially due to the high potential for pancreatic fistula with this procedure. However, many lesions including pancreatic neuroendocrine tumors (pNETS) and mucinous cysts may not require extensive pancreatic resections such as PD or DP, allowing for preservation of pancreatic endocrine and exocrine function. Given the relative infrequency with which this procedure is performed, few large series exist for minimally invasive approaches. Since the first reported laparoscopic CP reported by Baca and Bokan for a cystic pancreatic lesion [41], the number of series of minimally invasive CP continues to slowly increase (Table 27.4). In a review of 51 published cases, Machado et al. found the laparoscopic approach to be feasible, with similar rates of complications as compared to the open approach, with fistula being the primary source of clinically significant morbidity [46]. However, due to the technical complexity of this procedure, it remains limited to few specialized centers.
Table 27.4
Comparison of outcomes of robotic central pancreatectomy
Series | Year | Patients (n) | EBL (ml) | Time (min) | Conversion (%) | 30-day mortality (%) | Pancreatic fistula (%) | Length of stay (days) | R0 resection (%) | Lymph nodes (n) | Endocrine/exocrine insufficiency (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
Giulianotti [42] | 2010 | 3 | 233 | 320 | 0 | 0 | 33.3 | 9–27 | NR | NR | 0 |
Kang [43]
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|