
Risk Factors and Strategies for Prevention of Breast Cancer
 ABSTRACT
ABSTRACT
Breast cancer is the most frequently diagnosed cancer in women in the United States and the second most common cause of cancer death. Recent developments in the ability to assess and modify breast cancer risk represent an added hope to lessen the burden of this disease on individuals and societies. Multiple risk factors for breast cancer have been identified and include demographic factors reflective of endogenous hormone exposure, modifiable lifestyle factors, such as physical activity and alcohol intake as well as nonmodifiable factors including histologic lesions, genetic mutations, and family history. Randomized phase III chemoprevention trials established a beneficial effect of tamoxifen and raloxifene in preventing estrogen receptor-positive breast cancers, and the overall quality of life was similar in patients receiving these agents. Aromatase inhibitors are a promising option for breast cancer prevention and definitive studies are ongoing. Further research is needed to improve accuracy of breast cancer risk assessment and develop chemoprevention measures that will be more acceptable to women and reduce the risk of other breast cancer subtypes.
Keywords: breast cancer, risk assessment, risk reduction, tamoxifen, raloxifene, prevention
Breast cancer is the most frequently diagnosed cancer in women in the United States and the second most common cause of cancer death in this group. The chance of a woman developing breast cancer during her lifetime is approximately 1 in 8, and the chance of dying of breast cancer is 1 in 35 (1). It is estimated that 192,370 women were diagnosed with breast cancer in 2009, whereas more than 40,000 women died of the disease in the same year. Fortunately, the breast cancer death rate has been decreasing, most likely because of advances in early detection and treatment (2,3). Recent developments in the ability to determine and modify breast cancer risk represent an added hope to lessen the burden of this disease on individuals and societies. We examine the main approaches to assess and reduce the risk of breast cancer.
 BREAST CANCER RISK ASSESSMENT
BREAST CANCER RISK ASSESSMENT
There are a number of factors that may affect a woman’s risk of developing breast cancer. Broadly these can be grouped into demographic factors, lifestyle factors, and histologic lesions. Additionally, mammographic density (MD) (4,5) and genetic mutations (6) are independent risk factors. Statistical models have been developed to synthesize these factors and predict overall risk. The most appropriate model to predict risk is based on the clinical scenario.
 DEMOGRAPHIC RISK FACTORS
DEMOGRAPHIC RISK FACTORS
Age
The incidence of breast cancer increases rapidly with age until the age of 50, the average age at menopause, and then it increases at a slower rate (7,8). Breast cancer is still an uncommon disease in young women, with the probability of a diagnosis of 1 in 208 by age 39, versus 1 in 16 for women aged 70 and older (9). The median age of diagnosis is 61 years (10).
Race
Data from the Surveillance, Epidemiology and End Results program (SEER) demonstrate that, on the whole, incidence of breast cancer is lower in African American women compared with white women (10). The rate of breast cancer per 100,000 persons was 118.3 in African American women, whereas in white women it was 133.2. Potential reasons for this difference include a later age at first live birth and more frequent use of postmenopausal hormone replacement therapy (HRT) in white women (11). However, in younger women (age <40), African American women have a higher incidence of breast cancer than white women (16.8 vs 15.1/100,000 women years) with an increased prevalence of hormone receptor-negative and HER2-negative breast cancer (12,13). The reasons for this are unknown, but risk factors appear to vary with breast cancer subtype (14).
 RISK FACTORS REFLECTIVE OF ENDOGENOUS HORMONE EXPOSURE
RISK FACTORS REFLECTIVE OF ENDOGENOUS HORMONE EXPOSURE
Age of Menarche
Early menarche is a risk factor for breast cancer. A large international multicenter case-control study demonstrated that for each 2-year delay in menarche, the lifetime risk of breast cancer decreased by 10% (95% confidence interval [CI] 6–15) (15). Although some studies showed that the greatest effect of early menarche may be in increased rates of breast cancer in premenopausal women, most evidence indicates that the elevated risk affected all age groups equally (16).
Age at First Live Birth
Late age at first full-term pregnancy is an established risk factor for breast cancer. Data from the Breast Cancer Detection and Demonstration Project (BCDDP) demonstrated that risk of breast cancer increases four- to fivefold in women who have their first live birth after the age of 30 (P < .001). Moreover, the risk continues to increase with age at first live birth through the ages of 34 to 35 (relative risk [RR] 4.9; Ptrend < .001) (17).
Endogenous Hormone Levels
To estimate the effect of endogenous hormone levels on the risk of breast cancer in postmenopausal women, Key et al. (18) combined data from nine prospective studies including 663 women who developed breast cancer and 1765 women who did not. The RR of breast cancer was higher in women in the highest quintiles of estradiol (RR 2.00; 95% CI 1.47–2.71) or testosterone (RR 2.2; 95% CI 1.59–3.10) compared with those in the lowest quintiles. Increased risk was also noted for free estradiol, estrone, estrone sulfate, androstenedione, and dehydroepiandrosterone and is mitigated by sex hormone-binding globulin (SHBG).
Exogenous Hormones
Postmenopausal HRT
In the combined HRT Women’s Health Initiative (WHI) trial (N = 16,608) in which postmenopausal women were randomized to conjugated equine estrogen and progesterone (CEE+P), the hazard ratio (HR) for breast cancer was 1.26 (95% CI 1.00–1.59) and exceeded the predefined safety limits, resulting in early trial closure (19). Although baseline breast cancer risk factors were similar between the two arms (Gail risk model scores, family history [FH], race/ethnicity, obesity, and alcohol use), breast cancers diagnosed on HRT were more often at advanced stage (regional/ metastatic 25.4% vs 16.0%) and slightly larger(19). There were no differences in tumor histology, grade, or hormone receptor status. In the WHI CEE trial (20), CEE use was associated with a nonstatistically significant decrease in breast cancer incidence (HR 0.77; 95% CI 0.59–1.01). The effect of estrogen (E) alone on breast cancer risk in epidemiologic studies has been inconsistent (21,22). Taken together, the data suggest that E+P is more capable of inducing breast tumor growth than E alone.
Oral Contraceptive Pills
A reanalysis of 54 studies evaluating hormonal contraceptives and breast cancer risk determined that current users and those within 10 years of stopping oral contraceptive pills (OCP) had a small increase in risk (RR 1.24; 95% CI 1.15–1.33). Ten years after stopping, however, there was no increased risk (23). Additionally, formulations with higher E and P potency impart larger risk than newer OCP with lower hormone dose/potency (24).
 LIFE STYLE RISK FACTORS
LIFE STYLE RISK FACTORS
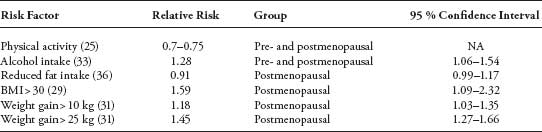
The association between lifestyle factors and breast malignancies has been investigated in multiple studies. Results are difficult to interpret because of differences in study designs, measurement errors, and variation in timing of exposure and follow-up. There is, however, convincing evidence that some modifiable lifestyle factors influence the incidence of breast cancer. Table 1 summarizes the impact of the various life style risk factors on the incidence of breast cancer.
Physical Activity
Physical activity (PA) has been associated with a reduction in breast cancer incidence. A recent meta-analysis evaluated the published literature on PA and breast cancer (25). The majority of the 62 studies demonstrated a 25% to 30% average breast cancer risk reduction in women who are physically active. Moreover, 28 of 33 studies showed a dose-response effect. The benefit was greater in postmenopausal women, especially those with a normal body mass index (BMI). Hormone receptor-negative tumors were reduced to a greater extent than hormone receptor-positive tumors. The mechanism underlying the protective effect of PA is unknown, and current hypotheses include change in sex steroid hormones and/or adipokine levels (26,27). The insulin, immune, inflammatory, and antioxidant pathways have also been proposed as mediators of effect (28)
TABLE 1
Impact of various life style risk factors on the incidence of breast cancer
Body Weight
BMI (kg/m2) is positively associated with risk of breast cancer in postmenopausal women. The European Prospective Investigation into Cancer and Nutrition (EPIC) study was designed primarily to investigate the relation between cancer and nutrition and evaluated more than 176,000 women (29). At 4.7 years median follow-up, this study showed that postmenopausal obese women (BMI > 30) had a 31% (Ptrend ≤ .002) increase in risk compared with women with BMI <25 (29,30). Conversely in premenopausal women, obesity may be protective against breast cancer (Ptrend = .0007). Regarding weight change, the EPIC study (31) showed an association between weight gain (15–20 kg) and breast cancer incidence in postmenopausal (RR, 1.50; 95% CI 1.06–2.13), but not in premenopausal women. In postmenopausal women, the association was present only in those who were not using HRT (Ptrend ≤ .0002). Change in serum sex hormones may provide the causal link. Overweight postmenopausal women have higher plasma levels of estrogen as a result of aromatization of androgens in peripheral fat and decreased SHBG. Use of HRT may obscure the effect of adiposity on breast cancer risk by altering the levels of steroid hormones (30). Overweight premenopausal women, however, have more irregular menstrual cycles and increased rates of anovulatory cycles resulting in less systemic exposure to estrogen and progesterone (30).
Alcohol
A recent meta-analysis of 98 trials reported that women who drank alcohol had a 22% (95% CI 9–37) higher risk of developing breast cancer (32). The authors estimated that for every additional alcoholic beverage consumed per day, the risk of breast cancer increased by 10% (95% CI 5–15). No differences were noted by type of alcohol or menopausal status. It has been suggested that the increased risk of breast cancer may be limited to estrogen receptor (ER)-positive tumors (33,34), supporting the suggestion that alcohol may exert its effect through circulating estrogens (35).
Nutrition
The association between fat intake and breast cancer remains controversial. The WHI Dietary Modification trial (36) randomized more than 48,000 postmenopausal women, aged 50 to 79, to a dietary intervention that included reducing intake of total fat to 20% of energy and increasing consumption of vegetables and fruits to at least five servings daily and grains to at least six servings daily versus no dietary intervention. The study showed a nonsignificant trend toward a reduced risk for breast cancer (HR 0.91; 95% CI 0.83–1.01). A meta-analysis of case-control and cohort studies by Boyd et al. (37) demonstrated a statistically significant association between higher total fat (RR 1.13; 95% CI 1.03–1.25), saturated fat (RR 1.19; 95% CI 1.06–1.35), and meat intake (RR 1.17; 95% CI 1.06–1.29) and breast cancer. A prospective analysis of more than 280,000 preand postmenopausal women in the EPIC study did not show an association between fruits and vegetable intake and breast cancer (38). These results were confirmed in other studies (39,40) including greater than 370,000 participants.
Additionally, epidemiology studies evaluating associations between carbohydrates (41,42), fiber (43), micronutrients (44–46), phytoestrogens (47), dairy products (48), vitamin D (49), calcium(49), processed food (50) and breast cancer have not shown convincing evidence of an association, although there continues to be ongoing interest in exploring effects of dietary components.
 BENIGN BREAST DISEASE
BENIGN BREAST DISEASE
One model of carcinogenesis is the serial progression of normal cells to atypical cells to invasive cancer with accompanying genetic changes (51). In the breast cancer pathway, several high-risk lesions are characterized that increase a woman’s chance for developing invasive and noninvasive breast cancer. Hartmann et al. followed 9087 women registered in the Mayo Clinic Surgical Index and Pathology Index, a data base of women aged 18 to 85 who had undergone surgical excision of a benign breast lesion. At a median of 15 years follow-up, their RR of developing malignant breast disease (52) was 4.24 (95% CI 1.45–1.68) for lesions with atypia, 1.88 (95% CI 1.66–2.12) for proliferative changes without atypia, and 1.27 (95% CI 1.15–1.41) for nonproliferative changes. The risk persisted for at least 25 years after biopsy.
 MAMMOGRAPHIC DENSITY
MAMMOGRAPHIC DENSITY
MD reflects the proportion of fibroglandular tissue on mammogram. Compared with women with low MD, women with high MD have a fourfold risk of developing breast cancer (odds ratio [OR]4.7; 95% CI 3.0–7.4) (53). A meta-analysis of 42 studies examining the association between MD and breast cancer demonstrated that there exists a strong dose-response relationship between MD and breast cancer regardless of the technique of MD assessment or the population studied, that is, the higher the density the higher the risk (5). Some experts, however, have criticized the use of MD as a breast cancer prediction tool because of high reader variability and significant technical barriers to accurate MD assessment (54). MD is dynamic and increases with agents that increase breast cancer risk, such as HRT, and declines with agents that reduce risk, including tamoxifen, and thus may be a useful biomarker of effect in the study of potential prevention agents (20,55,56).
 GENETIC PREDISPOSITION
GENETIC PREDISPOSITION
Inherited mutations in tumor suppressor genes BRCA1 and BRCA2 are the most powerful predictors of the development of breast or ovarian cancer (57–60). Hereditary breast cancer accounts for only 5% to 10% of all breast cancer cases, yet individuals carrying mutations in one of these genes have a 40% to 80% chance of developing breast cancer (6,58–60). Because of their high lifetime risk, mutation carriers may be candidates for prophylactic surgery (mastectomy and/or oophorectomy) to reduce risk. Bilateral prophylactic mastectomy reduces the risk of breast cancer by 90% in BRCA1/2 mutation carriers (61). Prophylactic oophorectomy done before age 50 reduces the risk of breast cancer by 50% (62). In an attempt to further define the role of genetic predisposition in patients with breast cancer, five genome-wide association studies have been conducted in women with invasive breast cancer and demonstrated a possible association between breast cancer risk and novel single nucleotide polymorphisms (SNPs) (63–67). The presence of these SNPs was associated with increased OR for breast cancer ranging from 1.13 to 1.64. The most commonly implicated gene on which a variety of SNPs have been identified is FGFR2, which encodes a receptor tyrosine kinase and is overexpressed in some breast cancers (68). Since these SNP variants likely have low penetrance, may be common, and may need to present in combination, the studies evaluating them are complex requiring thousands of cases and controls. No study has yet evaluated SNPs in a prospective fashion.
 STATISTICAL RISK MODELS
STATISTICAL RISK MODELS
To synthesize the demographic and histologic information affecting risk of breast cancer, a number of risk models have been developed.
The Gail Model
The Breast Cancer Risk Assessment tool, often referred to as the Gail model (69), was derived from the BCDDP, a study conducted to assess the feasibility of mammographic screening (70). Risk factors incorporated into the Gail model include age at the time of evaluation, age at menarche, age at first live birth, history of prior breast biopsies, and number of first-degree relatives with breast cancer. The Gail model was subsequently modified to account for risk associated with a history of atypical hyperplasia and risk in African American women (71). The Gail model has been validated in multiple studies (72–75) and is accessible on line (http://www.cancer.gov/bcrisktool/Default.aspx). Bondy et al. (73)reported that the Gail model was accurate in predicting risk for women who were compliant with mammographic screening (observed-to-expected case ratio 1.12; 95% CI 0.75–1.12). Spiegelman et al. (74), however, found that the Gail model over-predicted risk for younger women. Rockhill et al. (72) demonstrated that the Gail model was reliable in predicting breast cancer incidence in populations studied; however, it was comparable with chance in predicting risk for individual patients (c-statistic 0.58). Moreover, the Gail model is not appropriate for women younger than 35 years, with a history of ductal carcinoma in situ (DCIS), lobular carcinoma in situ (LCIS), or a strong FH. It can be expected that the accuracy of a model will change with time, as breast cancer incidence changes. Recently, the Gail model was recalibrated using more up-to-date SEER data to improve its predictive accuracy (76).
 MODELS ASSESSING STRONG FH OF BREAST CANCER
MODELS ASSESSING STRONG FH OF BREAST CANCER
The Claus model (77) was developed using information from the Cancer and Steroid Hormone Study (CSHS). The CSHS is a case-control study conducted by the Centers for Disease Control and the National Cancer Institute to evaluate the effect of OCP on more than 4000 women aged 20 to 54 and diagnosed with breast cancer. The Claus model is geared toward identifying women at risk for breast cancer based on genetic predisposition using an autosomal dominant model of transmission. This model predicts individual risk by the number of first- and second-degree relatives with breast cancer and their age at diagnosis. The Claus model is used for women in the setting of a strong FH.
The Tyrer–Cuzick model (78) is based on data acquired from a number of different sources and was developed to include genetic risk based on FH as well as personal factors (age at menarche, parity, age at first live birth, atypical ductal hyperplasia, BMI). The Tyrer–Cuzick model has been validated and shown to consistently predict breast cancer better than the Gail and Claus model since it incorporates FH history, hormonal factors, and benign breast disease in a comprehensive fashion when tested in a population seen at FH screening clinic (79–82). Other breast cancer risk prediction models have been introduced. The BRCAPRO, Ford, and Couch models predict the risk of being a BRCA1 or 2 mutation carrier (6,83).
Recently, Wacholder et al. (84) investigated the addition of 10 common genetic variants associated with breast cancer to the usual demographic risk factors. The area under the curve improved from 58% to 61.8%. The authors concluded that including the genetic variants only produced a modest improvement in breast cancer risk prediction.
MD has been added to several risk prediction models (85–88). Typically, these models based on demographic risk factors performed slightly better when MD was included. For example (85), during 5.3 years of follow-up, the Tice model was well calibrated with an observed/expected ratio of 1.03 (95% CI 0.99–1.06). It had, however, modest discriminatory accuracy with a concordance index of 0.66 (CI 0.65–0.67). The increase in c-statistic with the addition of MD was modest. The three other models had similar results (86–88). The increase in c-statistics with the addition of MD was modest, ranging from 0.01 to 0.06. MD remains an investigational tool and is typically not reported on standard mammogram reports.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 INTRODUCTION
INTRODUCTION