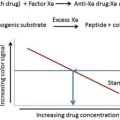
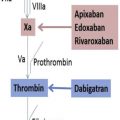
The vitamin K antagonists (VKAs) are associated with a significant rate of major and fatal bleeding complications. The new direct oral anticoagulants (DOACs), even though having a better bleeding profile than the VKAs, are still associated with serious bleeding. The anticoagulation induced by the VKAs can be reversed with both vitamin K and prothrombin complex concentrates, whereas the DOACs were developed without specific reversal agents. Although there is controversy around the necessity of a reversal agent, most clinicians agree that having a reversal agent for the DOACs would be beneficial. Three reversal agents are currently in development.
Key points
- •
The new direct oral anticoagulants have a bleeding risk profile better than the vitamin K antagonists, but are still associated with serious bleeding.
- •
The new direct oral anticoagulants were developed without specific reversal agents.
- •
Current recommendations for reversal of the new direct oral anticoagulants when major bleeding occurs include the use of prothrombin complex concentrates.
- •
There are 3 specific reversal agents in development for the direct oral anticoagulants, with one of them, idarucizumab, recently approved for clinical use.
Introduction
Oral anticoagulants, because of their intended function to impair coagulation and prevent or retard thrombus development, are associated with an almost obligatory incidence of bleeding ranging from nuisance bleeding to life-threatening and fatal bleeding. This bleeding incidence is well documented with the traditional oral anticoagulant, warfarin, one of the vitamin K antagonists (VKAs). This class of drugs is responsible for the most emergency hospitalizations due to an adverse drug event (bleeding), the most emergency room visits due to an adverse drug event, and the most common cause of death associated with an adverse drug event. Bleeding on warfarin is also an important contributor to the overall costs of anticoagulant therapy. Although most bleeding events occur when patients are therapeutically anticoagulated, VKA therapy is further complicated by poor management that allows patients to be over-anticoagulated and put at a higher risk of anticoagulant-related bleeding. Additional factors, such as poorly controlled hypertension and other comorbidities, further enhance the risk of bleeding, and better management of these comorbidities could lessen the incidence of bleeding. Given this serious potential side effect, it is only natural that one would like to be able to reverse anticoagulation when necessary, especially in the setting of life-threatening bleeding. However, having a reversal agent is not so simple. Questions arise as to how effective is the reversal, will reversal of anticoagulation impact overall outcome of the bleeding event, will reversal improve overall patient outcome, does the reversal agent have any deleterious side effects of its own, will reversal of anticoagulation place the patient at an undue risk of a thrombotic event, and will the reversal agent be expensive and possibly not cost-effective. Many of these questions are explored in the following discussion because such agents apply to the new direct oral anticoagulants (DOACs) as well as to warfarin.
Introduction
Oral anticoagulants, because of their intended function to impair coagulation and prevent or retard thrombus development, are associated with an almost obligatory incidence of bleeding ranging from nuisance bleeding to life-threatening and fatal bleeding. This bleeding incidence is well documented with the traditional oral anticoagulant, warfarin, one of the vitamin K antagonists (VKAs). This class of drugs is responsible for the most emergency hospitalizations due to an adverse drug event (bleeding), the most emergency room visits due to an adverse drug event, and the most common cause of death associated with an adverse drug event. Bleeding on warfarin is also an important contributor to the overall costs of anticoagulant therapy. Although most bleeding events occur when patients are therapeutically anticoagulated, VKA therapy is further complicated by poor management that allows patients to be over-anticoagulated and put at a higher risk of anticoagulant-related bleeding. Additional factors, such as poorly controlled hypertension and other comorbidities, further enhance the risk of bleeding, and better management of these comorbidities could lessen the incidence of bleeding. Given this serious potential side effect, it is only natural that one would like to be able to reverse anticoagulation when necessary, especially in the setting of life-threatening bleeding. However, having a reversal agent is not so simple. Questions arise as to how effective is the reversal, will reversal of anticoagulation impact overall outcome of the bleeding event, will reversal improve overall patient outcome, does the reversal agent have any deleterious side effects of its own, will reversal of anticoagulation place the patient at an undue risk of a thrombotic event, and will the reversal agent be expensive and possibly not cost-effective. Many of these questions are explored in the following discussion because such agents apply to the new direct oral anticoagulants (DOACs) as well as to warfarin.
When will reversal agents be needed?
The decision to use a reversal agent depends on many factors, including its documented or perceived effectiveness, cost, side-effect profile, and the risk of allowing thrombosis to break through when coagulation is corrected. An effective, inexpensive agent with minimal or no side effects would likely be used more often and for lesser degrees of bleeding than an expensive agent, or one that has potential side effects, or one that has questionable value in improving overall outcome. For reversal of warfarin anticoagulation, vitamin K might fit better in the former category, whereas prothrombin complex concentrates would fit better in the latter category. Given the current complexity and cost structure of reversal agents for the VKAs and the DOACs, it is likely that such agents will be used mostly for patients with major, hemodynamically unstable and life-threatening bleeding or bleeding into a vital organ. They could also be indicated for patients requiring emergent surgery or intervention who are currently on an anticoagulant or patients sustaining severe trauma who might be at great risk of life-threatening bleeding, but without detectable bleeding at the moment. Agents might be used in drug overdose and might even be considered to shorten the interval when an anticoagulant needs to be discontinued for an elective procedure in a patient with a strong underlying thrombotic risk.
Attributes of a useful reversal agent
In considering the DOACs and potential reversal agents, one would like an agent that is readily available to clinicians on the front lines, that acts rapidly to reverse anticoagulation and produces sustained normalization of coagulation, that has no significant side effects, does not induce a prothrombotic state, allows re-anticoagulation in the short term if needed, does not induce tolerance, and is relatively inexpensive or cost-effective ( Box 1 ). It should also have the potential to improve patient outcomes from the bleeding event, but whether the agent actually halts the bleeding or changes the clinical outcome is as much dependent on the source, size, and location of the bleed as it is on the reversal agent. Demonstrating improved outcomes will be difficult, if not impossible, in a randomized fashion when dealing with life-threatening bleeding.
- •
Readily available at the point of care
- •
Acts rapidly
- •
Produces sustained normalization of coagulation
- •
Has no significant side effects
- •
Does not induce a prothrombotic state
- •
Allows for re-anticoagulation after control of the acute bleeding event
- •
Does not induce tolerance and can be used repetitively, if needed, in the short or long term
- •
Does not induce an immune response
- •
Inexpensive or cost-effective
- •
Has the potential to improve patient outcome from the bleeding event
Do the vitamin K antagonists have a reversal agent?
The VKAs produce their anticoagulant effect by targeting a key enzyme in the vitamin K pathway, vitamin K oxide reductase complex 1, leading to a decrease in normal functioning vitamin K–dependent coagulation factors (factors II, VII, IX, and X and other vitamin K–dependent proteins). As a result, the prothrombin time (PT), expressed as an international normalized ratio (INR), and the activated partial thromboplastin time (aPTT) become prolonged with the INR being most reflective of therapeutic levels of anticoagulation. Reduced vitamin K will restore normal synthesis of the vitamin K–dependent factors.
The VKAs have a reversal agent, vitamin K, that meets many of the qualities noted above for an ideal agent: readily available, produces sustained normalization of coagulation, has no significant side effects (very rare anaphylaxis when administered rapidly), does not induce a prothrombotic state, does not induce tolerance, and is inexpensive. Unfortunately, its major limitation is that it takes 12 to 24 hours to achieve significant normalization of coagulation based on the time it takes for synthesis of the vitamin K–dependent coagulation factors. As such, it is impractical in the setting of major or life-threatening bleeding or need for urgent surgery. There may also be some degree of difficulty in re-anticoagulation depending on the dose of vitamin K used to restore coagulation.
Rapid correction of coagulation requires replacement of the vitamin K–dependent factors, which can be done by administering fresh frozen plasma (FFP) or prothrombin complex concentrates (PCCs), which contain high concentrations of the vitamin K–dependent coagulation factors (II, VII, IX, and X). FFP has several drawbacks, including high-volume infusions and the potential for fluid overload, longer preparation time, febrile and allergic reactions, hemolytic reactions, transmission of infection, and induction of transfusion-associated acute lung injury. PCCs are highly concentrated preparations of the vitamin K–dependent coagulation factors and have the advantage of being able to normalize coagulation within minutes of a brief infusion using small volumes of concentrate. Until recently, only 3-factor PCCs were available in the United States (containing very limited amounts of factor VII), but 4-factor PCC is now available. Both concentrates have been shown to rapidly correct the INR when compared with FFP, but there is still controversy about the potential to improve overall outcome. Sarode and colleagues recently evaluated the ability of 4-factor PCC compared with FFP to control bleeding and improve outcome in a randomized, open-label trial in 202 patients with warfarin-related bleeding. Although PCC more rapidly corrected the INR compared with FFP (62.2% of the PCC group vs 9.6% of the FFP groups achieved an INR of ≤1.3), overall patient outcomes were not significantly different (effective hemostasis was achieved in 72.4% vs 65.4% [95% confidence interval: −5.8 to 19.9] in the PCC vs FFP groups, respectively). There was no difference in red blood cell transfusion requirements, length of hospital stay, thromboembolic events, or deaths. Not surprisingly, there was a higher incidence of congestive heart failure in the FFP group (12.8%; median volume transfused = 813.5 mL) than in the PCC group (4.5%; median volume infused = 99.4 mL).
The major safety concern of PCCs is their potential for creating a prothrombotic state and inducing thromboembolism. Several studies have documented this potential, and in a meta-analysis of 27 studies (1032 patients) of 3- or 4-factor PCCs to reverse VKA-induced coagulopathy (almost all retrospective or prospective cohort studies), Dentali and colleagues found a 0.7% and 1.8% incidence of thromboembolism, respectively. This low rate must be balanced against the benefits of reducing major bleeding, but such proof will be difficult to demonstrate outside of randomized trials, which are difficult to conduct in these high-risk patients.
Regardless of the controversy over the benefit and value of reversal agents for the VKAs, guidelines generally recommend the administration of vitamin K and 4-factor PCCs to rapidly correct the coagulopathy as measured by the INR in the setting of major bleeding ( Table 1 ).
| Parameters | Vitamin K | Fresh Frozen Plasma | 3-Factor PCC | 4-Factor PCC |
|---|---|---|---|---|
| Constituents | Vitamin K | Vitamin K–dependent clotting factors | Factors II, IX, X and proteins C and S | Factors II, VII, IX, X and proteins C and S |
| Administration | Orally or IV | IV | IV | IV |
| Dose | 5–10 mg | 10–15 mL/kg | 25–50 IU/kg | 25–50 IU/kg |
| Onset of effect | 6–8 h | Duration of infusion | 15–30 min | 15–30 min |
| Adverse effects | Rare anaphylaxis | Fluid overload; febrile and allergic reactions; viral transmission; transfusion-related acute lung injury | Thromboembolic complications | Thromboembolic complications |
| Cost | Minimal | Moderate | High | High |
Current state of reversal of direct oral anticoagulants before the advent of specific agents
In the last 2 decades, research on oral anticoagulants has focused predominantly on direct neutralization of 2 specific coagulation factors in the final pathway of coagulation: thrombin (fIIa or activated prothrombin) and fXa (activated factor X). Although ximelagatran (a direct thrombin inhibitor) was the first marketed product of this research, it experienced only a brief lifespan on the European market and was shelved because of hepatic toxicity. Subsequently, 4 new target-specific, direct, oral anticoagulants have been developed, tested, and introduced to the market since 2009: dabigatran etexilate (factor IIa inhibitor), rivaroxaban, apixaban, and edoxaban (all factor Xa inhibitors). They were first shown to be effective in the primary prevention of venous thromboembolism in patients undergoing major orthopedic surgery and are approved for this indication in various countries, but their major indications are for stroke prevention in atrial fibrillation and the initial treatment of acute venous thromboembolism as well as the extended treatment of venous thromboembolism with some agents. Because these agents have a rapid onset and offset of action, a predictable dose effect, minimal drug-drug interaction or dietary interaction, they were developed without the need for monitoring or a specific reversal agent. Although they are shown to have an overall better bleeding risk profile than the VKAs, they are still associated with a clinically significant incidence of major bleeding. Their major advantage in reducing bleeding is related to a consistent reduction in intracranial hemorrhage compared with the VKAs. As a result, they are associated with a reduced rate of fatal bleeding, but site-specific fatal bleeding is not significantly different compared with the VKAs.
There is suggestive evidence that DOAC-related major bleeding, even without a reversal agent, results in outcomes no different than those seen with warfarin-related major bleeding where reversal agents are available. Majeed and colleagues, in an analysis of major bleeding from 5 phase 3 trials of dabigatran, showed that outcome was no different in patients who bled on warfarin versus those on dabigatran, where warfarin has a reversal agent and dabigatran does not. Similar results were seen by Piccini and colleagues, who analyzed results from the ROCKET-AF trial of patients who experienced bleeding on warfarin versus rivaroxaban.
Managing patients with DOAC-associated bleeding requires discontinuation of the anticoagulant, efforts to control bleeding if the site is known, administration of general volume support, red blood cell transfusions as needed, and platelet transfusions if platelet dysfunction or thrombocytopenia is present. For patients with recent drug ingestion, gastric lavage with activated charcoal is recommended (within 2–3 hours). Dabigatran, because of its lower protein binding, is amenable to hemodialysis (about 50%–60% of the drug can be removed), whereas the fXa inhibitors are not. For minor or moderate bleeding, these measures are adequate, but for life-threatening bleeding, it is currently advised to administer a prohemostatic agent such as PCCs. This recommendation is not based on robust clinical trials, but on evidence acquired from animal studies, reversal of drug activity in healthy individuals, and anecdotal case reports. These studies often produce conflicting results because of different animal models, different prohemostatic agents, and most importantly, different coagulation assays used to measure impaired coagulation. In healthy individuals, Eerenberg and colleagues showed that nonactivated 4-factor PCCs reversed the elevated PT and low endogenous thrombin potential induced by rivaroxaban, but it was ineffective in correcting the elevated thrombin time or ecarin clotting time induced by dabigatran. Marlu and colleagues showed that activated PCC (factor eight inhibitor bypass activity) best corrected several rivaroxaban-induced coagulation assays, while others have shown that PCCs can correct dabigatran-induced bleeding in animal models and may be effective in real life bleeding.
A recent study did show that PCCs can reduce active bleeding induced by the fXa inhibitor edoxaban. Zahir and colleagues performed punch biopsies in human volunteer subjects and assessed the ability of 4-factor PCC to reduce bleeding. Subjects were pretreated with a 60-mg dose of edoxaban before escalating doses of 4-factor PCC were administered. A dose of 50 IU/kg of PCC reversed the duration of bleeding and endogenous thrombin potential in all subjects, but there was a nonsignificant trend in reduction in bleeding volume.
In summary, DOAC-associated major bleeding can be managed with supportive care, fluid resuscitation, red blood cell transfusions, and, if life-threatening, 4-factor prothrombin concentrates (activated or nonactivated). Current recommendations for the management of bleeding in patients on DOACs are summarized in Table 2 .