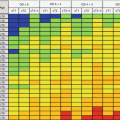
Low risk
Intermediate risk
High risk
Clinical T1–T2a
and Gleason <7 and PSA <10 ng/mL
Clinical T2b or Gleason 7 or PSA 10–20 ng/mL
Clinical T2c, T3a-b or Gleason 8–10 or PSA > 20 ng/mL
Localized
Locally advanced
RP, compared to watchful waiting (WW), had better survival rates in prospective randomized Scandinavian Prostate Cancer Group Study [4]. The benefit of RP on disease-specific mortality and metastasis rates was persistent beyond 10 years [5]. Overdiagnosis has been a fulcrum for follow-up. Despite the reported 50% overdiagnosis rate, recent other studies estimated this rate to be 23–28% [6, 7]. On the other hand, clinicopathological studies interestingly reported an overdiagnosis rate of 7–20% and claimed that underdiagnosis occurs more frequently than overdiagnosis [8, 9]. These results showed that overdiagnosis rates are much less than what’s believed. Currently there is no method to diagnose clinically insignificant prostate cancer, and in order to lower cancer-related deaths, it’s essential to ignore the low overdiagnosis rates.
An ideal candidate for RP is the one with a life expectancy of at least 10–15 years and with less comorbidity. Complete surgical resection is a must for an effective surgery. A palpable nodule on examination, positive imaging findings, and high percentage of positive biopsy cores are significant risk factors for extracapsular extension (ECE) [10, 11]. RP offers excellent progression-free survival rates when the tumor is organ confined even for the patients with high Gleason scores [12]. Excellent continence and erectile function rates are feasible with adequate experience. Furthermore, RP improves lower urinary tract symptoms and quality of life (QoL) parameters [13].
13.3 Indications of Nerve-Sparing (NS) Approach
Preoperative erectile function is the most important denominator of postoperative erection. Meticulous bilateral nerve-sparing approach in a young patient would result in satisfactory erections postoperatively. Walsh et al. were the first to show the possibility of sparing neurovascular bundle (NVB) that contains the cavernous nerves. Recently, many authors reported their outcomes about this technique [14].
Although NS technique can safely be performed in localized disease, indications of nerve-sparing RP are still debatable [15]. Preserving cavernous nerves without jeopardizing oncological principles may only be possible with a sufficient learning curve. For the beginner surgeons at the onset of this curve, clinicopathological risk factors demonstrating ECE may guide them to better select candidates. Nowadays certain nomograms are widely used in order to predict ECE [16–19]. Similarly new-generation MRI technology may give an insight to predict ECE and locally advanced disease [20–22], although MRI is not sensitive enough to find all tumors with extra-prostatic growth or microscopic ECE [23, 24]. The use of MRI in low-risk patients has no benefits either [25, 26].
Undoubtedly the most critical decision to perform NS approach is made during the surgery. When the tumor extends beyond the capsule, it’s possible to resect the prostate with negative surgical margin by interfascial dissection [27]. But NVB must be resected in case of invasion or suspicion of residual tumor inside it on palpation.
13.4 Steps in Retropubic Radical Prostatectomy
An 8–10 cm midline incision is made through subcutaneous tissues between the umbilicus and pubis symphysis. After dissecting the rectus muscles, the transverse fascia will sharply be opened at midline (Fig. 13.1). Blunt dissection starting laterally the peritoneum will be released cranially up to iliac vessels. The fat anterior and lateral to the prostate is teased away to expose the underlying anatomy. Endopelvic fascia is incised laterally between the prostate and the levator ani muscles (Fig. 13.2).
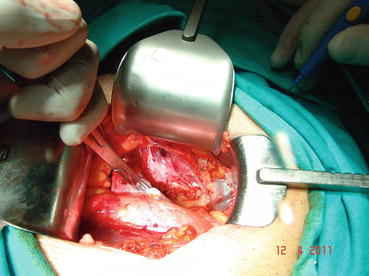

Fig. 13.1
Midline incision and exposure of retzius space

Fig. 13.2
Endopelvic fascia is incised
In most patients, an opening is found in the endopelvic fascia laterally of the puboprostatic ligaments. An incision is made from that opening throughout the lowest point of the fascia. The puboprostatic ligaments can then be sharply incised at their attachment to the pubic bone, with care taken to not damage the dorsal vein complex (DVC) (Fig. 13.3). A figure-of-eight suture is then placed approximately 1 cm cephalad to the anterior bladder neck, to ligate the superficial DVC. This suture helps identifying the proper location to divide the bladder neck later in the surgery and also to minimize backflow bleeding (Fig. 13.4). The deep DVC is ligated distally after the DVC is isolated from the urethra just beyond the apex of the prostate (Fig. 13.5). The fascial layer surrounding the DVC and urethra is bluntly ruptured with the index finger, allowing the surgeon to appreciate a sulcus between the most distal part of urethra and the DVC. Gentle traction on the Foley catheter may help identify the urethra. A long, blunt-tipped right-angle clamp is passed between the DVC and the urethra and is used to grasp stainless steel wire. The wire loop will be used as a guide transecting the DVC, after the distal suture ligature is placed through the DVC, a few millimeters dorsal and caudal to the wire (Fig. 13.6). The suture is anchored through the posterior periosteum of the pubis and tied securely, controlling bleeding from the DVC when it is divided. After releasing the urethra from the fibrous fibers adjacent to the urogenital diaphragm posteriorly and lifting it with a right-angle clamp, the urethra is cut 270° (Fig. 13.7). Then, 3.0 Vicryl anastomotic stitches are placed at 1 and 11 o’clock positions. Then bilateral 3, 5, 7, and 9 o’clock sutures are placed (Fig. 13.8). After passing six stitches from the urethra, the catheter is held with a thick clamp and cut off, the distal part is removed from the urethra, and the proximal part is directed cranially without excessive traction. At this point, the posterior 90° part of the urethra is cut off, and the urethra is completely separated. Subsequently, rectourethral fascia (posterior striated sphincter) will be dissected. This stage is extremely important because finding the exact plane is essential both for the negative surgical margin and for preventing rectal injury.
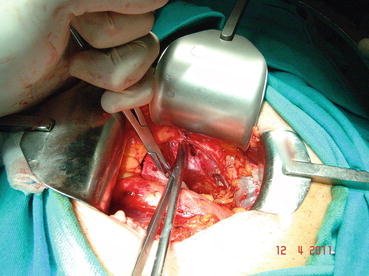
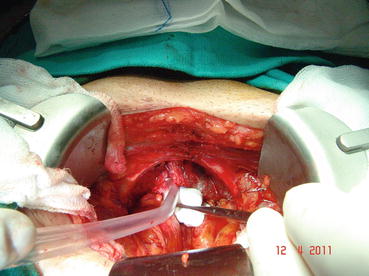
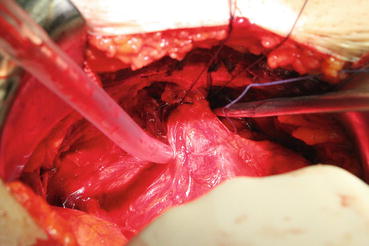
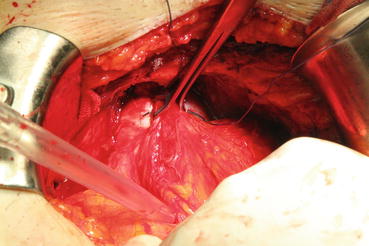
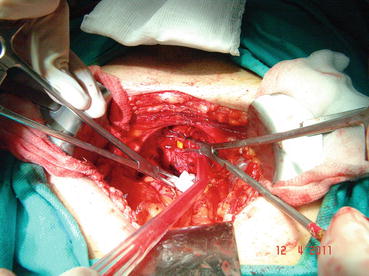
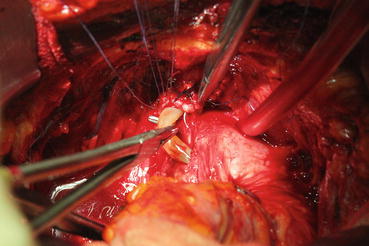

Fig. 13.3
Puboprostatic ligament is cut

Fig. 13.4
Dorsal vein complex

Fig. 13.5
Dorsal vein complex is ligated

Fig. 13.6
Backflow suture

Fig. 13.7
Urethra is cut with 270°

Fig. 13.8
Urethra sutures
The cavernous nerve passes laterally to the rectourethral fascia . Without dissecting this tissue, which is relatively thin in some patients and scarred in others due to the possible desmoplastic reaction, the posterior part of the prostate will not be liberal. The correct depth for the dissection border can be defined as the appearance of pararectal fat tissue (Fig. 13.9). Seminal vesicles will appear when the lateral pedicle is fully ligated. A single clamp cannot control the lateral pedicle. The lateral pedicles are separated after multiple ligations of the vascular and fibrous ligaments with individual clips or via clamps. The lateral dissection is continued until the junction of the seminal vesicle with the prostate floor. Opening the bands between the rectum and the Denonvilliers’ fascia covering the seminal vesicle continues the dissection. At this point, both the vas deferens and seminal vesicles emerge. First, the vas deferens is separated, clipped, and cut. The fibrous adhesions between the distal part of the vas deferens and the seminal vesicles are separated so they are clearly revealed. After this point, the end of the seminal vesicles is reached with blunt and sharp dissection. Here, the seminal vesicle artery is clearly displayed and clipped, which allows mobilization of the seminal vesicle (Fig. 13.10).
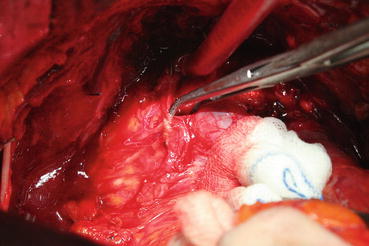
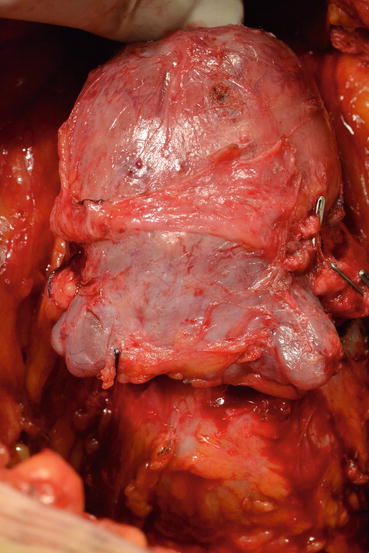

Fig. 13.9
Pararectal fatty tissue

Fig. 13.10
Seminal vesicles mobilized
Later, the dissection is directed toward the bladder neck in the posterior plane (Fig. 13.11). After finding the bladder neck, separation of the prostate will continue anteriorly (Fig. 13.12). After observing the orifices, the posterior part of the bladder neck is cut with scissors and taken out. At this point the catheter will be removed with the specimen (Fig. 13.13). The bladder neck is closed posteriorly in a tennis racquet fashion. The mucosa is everted with fine 3.0 Vicryl sutures (Fig. 13.14). When adequate hemostasis has been achieved, 20 F Foley catheter will be advanced into the bladder and inflated with 10–15 cc sterile water. The six sutures placed in the urethra are passed through the appropriate points on the bladder neck—from inside to outside. The catheter is placed on traction to bring the bladder neck down to the urethra, slack is taken out of the sutures, and the bladder is gently retracted with the anastomotic sutures tied in an anterior-to-posterior fashion. A sump drain is then placed through the anterior abdominal wall through a separate stab incision, and then the fascia and skin will be closed.
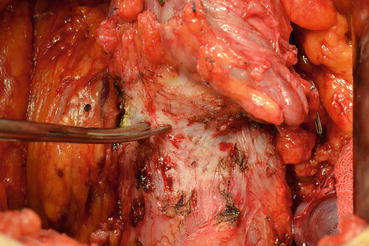
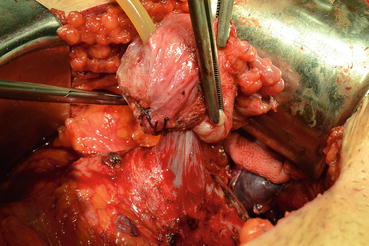
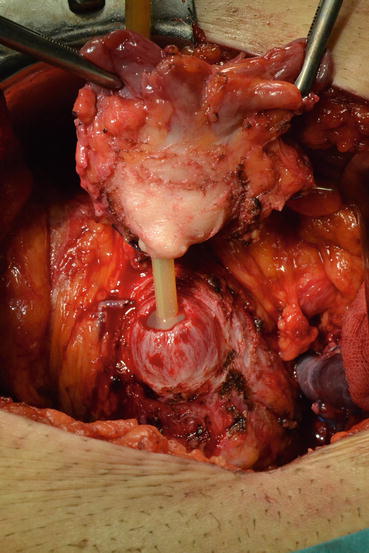
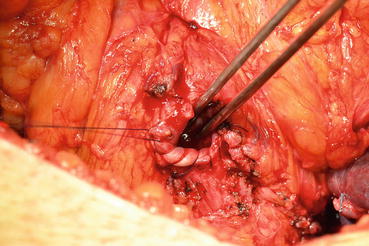

Fig. 13.11
Bladder neck is dissected

Fig. 13.12
Anterior dissection of prostate

Fig. 13.13
Prostate is removed

Fig. 13.14
Bladder neck mucosa is everted
13.5 Nerve-Sparing Technique
To preserve nerves, cautery usage should be avoided. Instead, crossing vessels passing to the prostate should be ligated with clips [28] (Fig. 13.15). As cavernous nerves pass between the prostatic and levator fascia, the prostatic fascia should not be harmed. The levator fascia should be opened as much as cranially from the bladder neck level from the thickened part. This approach is preferred, since it will also help to mobilize the prostate. If you start to spare nerves gently from prostatic apex and continue caudally, the rest of procedure will be much easier.
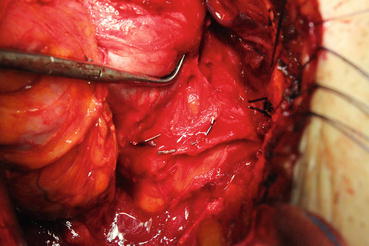

Fig. 13.15
Nerve-sparing technique
13.6 Complications
Several factors may complicate the surgery in short or long term. Those are mainly the surgeon’s experience, patient age, comorbidities, prostate and tumor volume, pelvic anatomy, and neoadjuvant hormone or radiotherapy. In other words, patient selection is the key point here. RP was not so popular for many years mainly because of excessive bleeding [29]. Understanding the anatomy has significantly decreased both the amount of bleeding and transfusion rates. An old study has shown a mean blood loss of 1100 (800–1600) cc during RP [30]. A recent meta-analysis with 167.184 open RP patients reported a median blood loss to be 750 cc [31]. Dorsal vein complex is the main cause of bleeding and so it should be sutured carefully. Nerve-sparing approach may also increase bleeding.
Rectal injury is a rarely reported complication and is directly related to surgical experience and technique [31, 32]. Most surgeons do routine preoperative mechanical bowel preparation besides antibiotics. So they can easily close defect primarily in case of rectal injury. A meta-analysis including 35.099 RP patients reported 0.43% rectal injury rate [33]. Of those with rectal injury, 48% received preoperative mechanical preparation. Infection, delayed colostomy, and length of stay rates were not statistically different between mechanical and nonmechanical bowel preparation groups.
Lymphocele is a relatively common complication for patients who underwent lymphadenectomy. When lymphatics are well controlled by cautery, clips, or sutures, the risk will be minimal. The rate of symptomatic lymphocele requiring intervention was reported to be 1.1–9.1% [34]. However, this rate increases to 27–61% when asymptomatic lymphoceles are also included [35]. Conservative management is the best option for asymptomatic patients. In case of pain or fever, USG or CT-guided drainage and antibiotics are required.
Postoperative infection is very rare and when it occurs is mostly wound infection. Postoperative antibiotics are not required if there are no symptoms. Single-dose second-generation cephalosporin is preferred for prophylaxis at anesthesia induction. Nevertheless, antibiotics could be beneficial when excessive intraoperative bleeding or long-standing postoperative drainage has occurred especially for obese and diabetic patients.
Deep vein thrombosis (DVT) is undoubtedly the most important etiology of 1% mortality observed in RP. Catalona reported 1.3% thromboembolic complication rate [36]. DVT can be prevented by shortening duration of surgery to less than 2 h and early postoperative mobilization and using air compression socks [37]. Low-molecular-weight heparin derivatives such as enoxaparin sodium may be given for high-risk patients.
13.6.1 Erectile Dysfunction
There are several studies related to this subject in limitation. Although there are many valid surveys, there is variable data related to ED. This is related to the patient group heterogeneity and the different erection reporting by the physician and the patients. While physicians always have good results, the situation may be different on the patients’ side. This shows that erectile function is a concept assessed and rated relatively not through yes-or-no questions.
Diabetes, hypertension, and drugs used for these reasons may cause adverse effects on the patient’s erectile function in the preoperative period and also may cause poor outcome after RP. Another important subject is the preoperative erectile function. It is considered one of the most important factors predicting erection recovery [38].
In his series Catalona followed up 3477 patients treated with RP for 18 months [36]. Erections sufficient for intercourse occurred in 76% of preoperatively potent men treated with bilateral nerve sparing and 53% of men treated with unilateral or partial nerve sparing surgery. Younger age and bilateral nerve sparing were significant factors for erection recovery. Huland et al. followed up patients for more than 12 months and reported erection recovery rate of 70% in patients younger than 55 years old with bilateral NS. They also found that this rate decreases to 37% in patients older than 65 with unilateral NS [39].
Another important factor affecting postoperative potency is the accessory pudendal artery. A study of 2399 patients who underwent bilateral NSRRP showed that accessory pudendal artery preservation provides a twofold advantage in preserving potency and leads to potency recovery sooner [40].
In a prospective, randomized controlled study, the postoperative 12-month potency status of 778 RRP and 1847 robot-assisted laparoscopic radical prostatectomy (RALP) patients with age < 75, PSA < 20, and T < 4 was compared using questionnaire forms [41]. ED was reported to be 74 and 70.1% in both RALP and RRP, respectively. However, it should be noticed that more lymph node dissections were performed with less nerve preservation in RRP group. The first results of a randomized controlled trial of 47 RRP and 22 RRP were published, and the potency rate in the RRP arm at the 12th postoperative month was 26%, and the same rate was found as 55% in the RALP arm [42]. In the study, a questionnaire form was used, and there was no difference between patients’ preoperative potency status and tumor characteristics. Krambeck et al. retrospectively studied the results of 403 RRP and 203 RALP after a 1-year follow-up [43]. NS approach was applied less in RRP group. There was no difference in the tumor characteristics between two groups. After 1 year they assessed erectile function by using questionnaire form, and no difference was found between the two groups’ potency status (RRP 63%, RALP 70%). The 12-month results of phase 3 study in which 163 RRP and 163 RALP were randomized were published [44]. There was no difference between the 12-month questionnaire form scores between the patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree