BACKGROUND
Diabetic retinopathy prevalence at baseline
Diabetic retinopathy, a microvascular complication of diabetes, remains a leading cause of acquired blindness in young and middle-aged adults.1 Currently, the estimated general population prevalence rates for retinopathy and vision-threatening retinopathy in the US are 3.4% (4.1 million persons) and 0.75% (899 000 persons), respectively.2 These pooled data focus almost exclusively on Type 2 diabetes.1 An epidemiologic study of subjects with Type 1 diabetes based on data from the New Jersey 725 Study and Wisconsin Epidemiologic Study of Diabetic Retinopathy, estimated prevalence rates of retinopathy at 1 per 300 persons aged 18 years and older and rates of vision-threatening retinopathy at 1 per 600 persons.3 Specifically, of the estimated 889 000 persons with Type 1 diabetes diagnosed before age 30 years in the US, 767 000 (86.4%) have some degree of retinopathy and 376 000 (42.1%) have vision-threatening retinopathy.3 These prevalence rates of diabetic retinopathy are particularly concerning given the increasing prevalence of diabetes mellitus.1
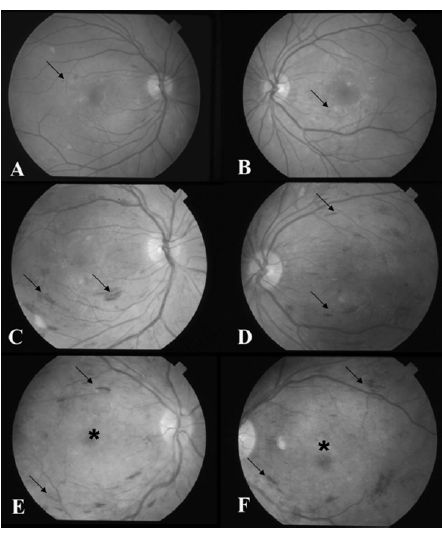
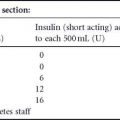
Fig. 17.1 During the first trimester, dilated examination of the (A) right and (B) left eye reveals occasional intraretinal hemorrhages (arrows) consistent with minimal non-proliferative diabetic retinopathy. (C and D) After institution of rapid glycemic control, the patient developed increasing intraretinal hemorrhages (arrows) indicative of severe non-proliferative diabetic retinopathy during the second trimester. (E and F) In the third trimester, examination revealed further retinopathy progression (arrows) with the development of clinically significant macular edema (*).
Pregnancy as a risk f actor for w orsening retinopathy
Pregnancy, with its hormonal, hemodynamic, metabolic, and immunologic changes, is a risk factor for progression of diabetic retinopathy. While the landmark studies focused on worsening retinopathy in pregnant Type 1 diabetic women,4–6 the findings of these studies can be extrapolated to pregnant women with Type 2 diabetes as the retinopathy seen in the two groups is essentially similar. Gestational diabetes, however, is not a risk factor for the development of retinopathy during pregnancy, but may be suggestive of a genetic risk for subsequent diabetes mellitus.7
OVERVIEW OF DIABETIC RETINOPATHY CLASSIFICATION
Diabetic retinopathy in both Type 1 and Type 2 diabetes is broadly classified as either non-proliferative or proliferative (Table 17.1). Non-proliferative diabetic retinopathy occurs when there are only intraretinal microvascular changes, such as microaneurysms and retinal hemorrhages (Fig. 17.2). In advanced non-proliferative diabetic retinopathy, progressive capillary non-perfusion of the retina may develop and lead to increasing ischemia, which results in the more severe proliferative phase. Pro-liferative diabetic retinopathy (Fig. 17.3) is characterized by new vessels on the retinal surface or optic disc that can bleed and result in the vision-threatening complications of vitreous hemorrhage, fibrotic scarring, and fractional retinal detachment. In both non-proliferative and proliferative diabetic retinopathy, increased retinal vascular permeability can result in accumulation of fluid in the retinal area serving central vision. This retinal thickening, known as macular edema (Fig. 17.4), is a leading cause of visual loss in diabetic patients.
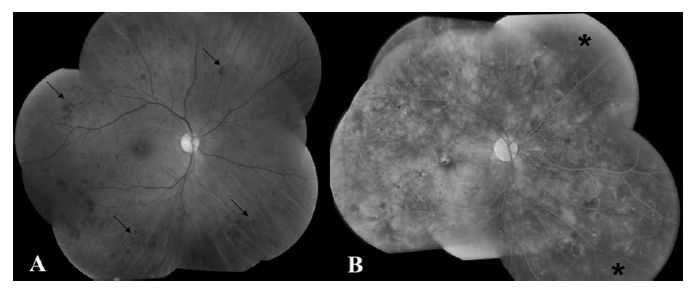
Fig. 17.2 (A) Fundus photograph of the right eye reveals dot-blot intraretinal hemorrhages in all four quadrants (arrows), consistent with severe non-proliferative diabetic retinopathy. (B) Fluorescein angiograghy shows patches of non-perfusion in the peripheral retina (*), indicative of the severe nature of the non-proliferative retinal changes.
Table 17.1 Classification of diabetic retinopathy.
| Classification | Lesions present |
| No retinopathy | No lesions present |
| Non-proliferative retinopathy | Intraretinal microvasculature changes only |
| Mild | Mild levels of microaneurysms and intraretinal hemorrhage |
| Moderate | Moderate levels of microaneurysms and intraretinal hemorrhage |
| Severe | Presence of one of the following features (4: 2: 1 rule): |
| • Severe intraretinal hemorrhage in all four quadrants | |
| • Venous beading in two or more quadrants | |
| • Moderate intraretinal microvascular anomaly (IRMA) in at least one quadrant | |
| Proliferative retinopathy | Neovascularization on the retinal surface |
RISK FACTORS FOR PROGRESSION OF DIABETIC RETINOPATHY
Diabetic retinopathy, a microvascular complication, is an end-organ response to a systemic disease. Concomitant systemic factors, therefore, influence the development and progression of diabetic retinopathy.8 A thorough understanding of diabetic retinopathy is necessary to discuss the specific retinal changes found in the diabetic pregnant patient. To do this, landmark studies regarding diabetic retinopathy in non-pregnant individuals will first be examined followed by a review of the specific studies focusing on diabetic retinopathy during pregnancy. A number of risk factors have been identified in large epidemiologic studies that are relevant to both pregnant and non-pregnant diabetic patients, although their role in the dynamic physiologic state of pregnancy is unique.
Glycemic control
Chronic hyperglycemia instigates a cascade of events leading to microvascular complications in diabetes. The landmark studies investigating glycemic control and its effects on diabetic complications include the Diabetes Control and Complications Trial (DCCT) and the United Kingdom Prospective Diabetes Study (UKPDS). Both of these clinical trials demonstrated the beneficial effects of intensive glycemic control in reducing the complications of diabetes.
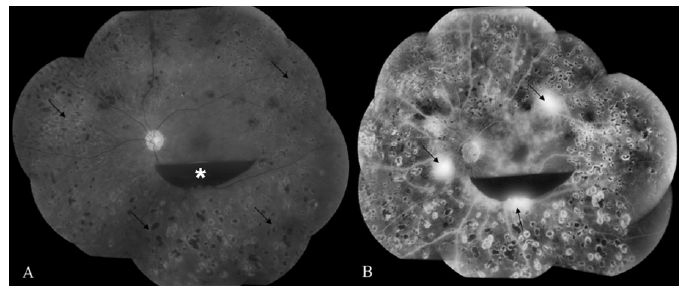
Fig. 17.3 (A) Fundus photograph of the left eye shows preretinal hemorrhage overlying the macula (*). The peripheral retina has many laser photocoagulation scars (arrows) indicative of previous treatment for proliferative diabetic retinopathy. (B) Fluorescein angiogram shows patches of bright hyperfluorescence (arrows) corresponding to areas of leaking neovascularization consistent with proliferative diabetic retinopathy.
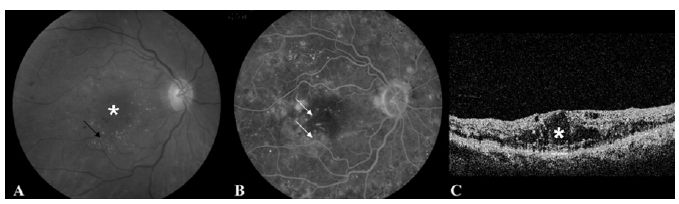
Fig. 17.4 (A) Fundus photograph of the right eye shows macular fluid (*) and lipid (arrow) consistent with clinically significant macular edema. (B) Fluorescein angiography reveals multiple leaking microaneurysms (arrows). (C) Optical coherence tomography confirms the presence of cystic fluid changes in the macula (*).
Diabetes Control and Complications Trial
The DCCT was a randomized, multicenter, prospective trial designed to determine if intensive glucose control, with the goal of near-normal HbA1c levels, would affect the development and progression of diabetic complications in Type 1 diabetes. The 1441 participants were randomly assigned to either conventional or intensive treatment for glucose control and followed for a mean duration of 6.5 years.9–13 The mean HbA1c was 7.2% in the intensive treatment group and 9.1% in the conventional control cohort. Intensive treatment resulted in a decreased risk of either the development or progression of diabetic retinopathy. One of the adverse effects of intensive therapy included initial worsening of retinopathy; however, this reversed after 18 months. In patients without any visible retinopathy when enrolled in the DCCT, the 3-year risk of developing retinopathy was reduced by 75% in the intensive treatment group compared with the standard treatment group. The benefit of the strict control was also evident in patients with existing retinopathy. There was a 50% reduction in the rate of progression of retinopathy compared with controls. When the DCCT results were stratified by HbA1c levels, there was a 35–40% reduction in the risk of retinopathy progression for every 10% decrease in HbA1c (e.g. from 8% to 7.2%).
United Kingdom Prospective Diabetes Study
The UKPDS,14 the largest and longest study of patients with Type 2 diabetes to date, was designed to evaluate the effect of conventional versus intensive glucose management on diabetic complications in 3867 subjects with newly diagnosed diabetes. The study confirmed that the beneficial effects of tight glycemic control on the incidence and progression of diabetic retinopathy also apply to patients with Type 2 diabetes. During a 9-year follow-up, the UKPDS showed a 25% reduction in the risk of the “any diabetes-related microvascular end point,” including the need for retinal photocoagulation, in the intensive treatment group compared with the conventional treatment group. For every percentage point decrease in HbA1c (e.g. 9% to 8%), there was a 35% reduction in the risk of microvascular complications.
Concomitant hypertension
Multiple studies have suggested that diabetic patients with concomitant hypertension are at increased risk for the development and progression of diabetic retinopathy, although the data are conflicting. Hypertension is thought to exacerbate diabetic retinopathy through mechanical stretching of endothelial cells, resulting in increased release of vascular endothelial growth factor (VEGF).15 Large studies correlating tight blood pressure control with reduced risk of retinopathy progression include the Wisconsin Epidemiology Study of Diabetes Retinopathy (WESDR), the UKPDS, and the Appropriate Blood Pressure Control in Diabetes (ABCD) trials.
Wisconsin Epidemiology Study of Diabetic Retinopathy
The WESDR was a 14-year population-based cohort study in southern Wisconsin assessing the prevalence and risk of diabetic retinopathy among 634 subjects with Type 1 diabetes, diagnosed before age 30 years.16 In addition to higher HbA1c and greater severity of retinopathy at baseline, hypertension was demonstrated to be a risk factor for the development of proliferative diabetic retinopathy. Furthermore, participants in the lowest quartile of systolic and diastolic blood pressure had significantly lower rates of progression to proliferative diabetic retinopathy compared with the highest quartile. These findings were independent of glycosylated hemoglobin.16
United Kingdom Prospective Diabetes Study
Of the 3867 Type 2 diabetic subjects in the UKPDS, a cohort of 1148 hypertensive subjects were selected of whom 758 were allocated to tight blood pressure control (defined as a blood pressure <150/85mmHg) and 390 to less tight control, with a median follow-up of 8.4 years.17 There was a 34% reduction in a two-step progression of retinopathy and a 47% reduction in the risk of moderate vision loss (≥15 letters) in the tight control group compared with the conventional group. The vision data, although not controlled for glycosylated hemoglobin, suggest that tight blood pressure control reduced the risk of diabetic macular edema, the primary cause of visual loss in Type 2 diabetes.
Appropriate Blood Pressure Control in Diabetes Trial
The ABCD trial also showed a correlation between tight blood pressure control and decreased risk of retinopathy.18–20 In the normotensive cohort of 480 subjects with Type 2 diabetes, there were no significant differences in glycosylated hemoglobin levels between patients randomly allocated to intensive or moderate antihypertensive therapy. For the last 4 years of follow-up, the mean blood pressure was 128/75 mmHg in the intensive treatment arm and 137/81 mmHg in the moderate group.19 During a 5-year follow-up period, there was less progression of diabetic retinopathy in the intensive blood pressure therapy group (34% in the intensive group compared with 46% in the moderate control group; p = 0.019).
Elevated serum lipid levels
Although the association between elevated serum lipids and diabetic retinopathy is uncertain, both the WESDR and Early Treatment Diabetic Retinopathy Study (ETDRS) found that elevated levels of serum lipids were associated with increased severity of retinal hard exudates.21,22 In the ETDRS, patients with total serum cholesterol levels of 6.21 mmol/L (240 mg/dL) or more were twice as likely to have hard exudates as those with levels less than 5.17 mmol/L (< 200 mg/dL). Low density lipoprotein (LDL) cholesterol levels paralleled the total serum cholesterol results, with subjects having almost twice the risk of developing hard exudates when serum LDL levels were 4.14 mmol/L (160 mg/dL) or more compared with levels less than 3.36 mmol/L (<130mg/dL). Hard exudates are significant because their severity at baseline in the ETDRS was associated with decreased visual acuity independent of accompanying macular edema. In fact, the strongest risk factor for the development of vision-threatening subretinal fibrosis in the ETDRS patients with diabetic macular edema was the presence of severe hard exudates.23 Of the 264 eyes with multiple hard exudates at baseline or during follow-up, subretinal fibrosis developed in 30.7%. In contrast, this complication only developed in 0.05% of the 5498 eyes with clinically significant macular edema but no severe hard exudates.23
Renal disease
Renal disease is a risk factor for retinopathy just as retinopathy is a risk factor for renal disease. In the WESDR, gross proteinuria was shown to be a risk factor for proliferative diabetic retinopathy.24 The study demonstrated that subjects taking insulin with gross proteinuria at baseline had approximately twice the risk of developing proliferative retinopathy over 4 years of follow-up compared with those without gross baseline proteinuria. Additionally, microalbuminuria is a marker for the risk of proliferative retinopathy. A cross-sectional examination of the WESDR data showed that younger-onset diabetic subjects (diagnosed before 30 years of age) and older-onset subjects (diagnosed aged 30 years of age or older) were both more likely to have retinopathy than those without microalbuminuria. This relationship remained true even after controlling for glycemia, hypertension, and duration of diabetes.25
RISK FACTORS FOR PROGRESSION OF DIABETIC RETINOPATHY DURING PREGNANCY
Early case–control studies reported that pregnancy is a risk factor for the progression of diabetic retinopathy,26,27 although these changes often regress postpartum.26 Several larger studies have since confirmed the transient progression of diabetic retinopathy during pregnancy without increased long-term risk.4,5 The mechanism of retinopathy acceleration is unknown, although multiple theories exist related to the hormonal, hemodynamic, metabolic, and immunologic changes associated with pregnancy. Reported risk factors for retinopathy progression in pregnant diabetic patients are described below.
Glycemic control
Diabetes in Early Pregnancy study
The Diabetes in Early Pregnancy (DIEP) study6 was a prospective cohort study of 155 diabetic women followed from the periconceptional period to 1 month postpartum. Acceleration of retinopathy was observed in 10.3% of patients with no retinopathy, 21.1% of those with microaneurysms only, and 18.8% of those with mild non-proliferative diabetic retinopathy. However, the greatest progression was noted in patients with moderate-to-severe non-proliferative diabetic retinopathy at baseline of whom 54.8% experienced a worsening of retinopathy. Proliferative diabetic retinopathy developed in 6.3% with mild and 29% with moderate-to-severe baseline retinopathy. Unlike earlier studies,26 the DIEP found that changes in metabolic control were more important then duration of diabetes in predicting retinopathy progression. Women in this study with the poorest pre-pregnancy glycemic control and the greatest reduction in HbA1c during the first trimester were at higher risk for retinopathy progression. While the DIEP study did not elucidate the mechanism for worsening retinopathy, it did indicate the clinical importance of optimizing metabolic control before conception to reduce the risk of retinopathy progression.
Diabetes Control and Complications Trial ancillary study
An ancillary study of the DCCT5 evaluated the role of pregnancy on diabetic retinopathy progression. Similar to the DIEP study,6 the DCCT study emphasized the importance of optimal glycemic control prior to pregnancy. In this study of 680 diabetic women, 180 women became pregnant. These pregnant women had a higher risk of retinopathy progression compared with the 500 non-pregnant study participants. In addition, the women in the conventional treatment group who did not have tight control prior to conception had a 2.48-fold greater risk of retinopathy acceleration during pregnancy compared with the non-pregnant group. By contrast, women in the intensive therapy arm who had tight control prior to pregnancy had only a 1.63-fold greater risk of retinopathy progression during pregnancy compared with the non-pregnant women. Therefore, tight metabolic control prior to conception is the ideal.
Hypertension
Hypertension during pregnancy is a risk factor for retinopathy progression. A prospective study of 154 diabetic women found that 55% of women with chronic or gestational hypertension suffered a deterioration in retinopathy compared with 25% of the pregnant diabetic women without hypertension.28 Another prospective study by Klein et al indicated that elevated diastolic blood pressure was a risk factor for progression of retinopathy, although not as strong a risk factor as glycemic control.27
Serum lipids
Although dyslipidemia has been associated with increasing macular exudates that can lead to the vision-threatening complication of subretinal fibrosis in general diabetic studies,21,22 the role of increased serum lipids in pregnant diabetic women has not been studied extensively. Nevertheless, because of the general health benefits of lipid control, optimizing cholesterol and lipids prior to pregnancy is recommended as this could reduce the risk of macular exudates.
Renal disease
As with serum lipid levels, renal disease indicated by proteinuria or microalbuminuria has not been examined in a controlled fashion in pregnant diabetic subjects. However, since microalbuminuria has been identified as a risk factor for retinopathy development in the general diabetic population, careful renal monitoring in pregnancy is prudent.24,25
POSSIBLE MECHANISMS OF DIABETIC RETINOPATHY PROGRESSION DURING PREGNANCY
Metabolic theory
The metabolic theory simply states that the rapid normalization of glycemia during pregnancy promotes acceleration of diabetic retinopathy. Pregnancy is a state where aggressive control of serum glucose has been routinely instituted, even prior to the results of the DCCT.6,28,29 The concept of rapid glycemic control resulting in a transient deterioration of retinopathy had previously been noted in non-pregnant diabetic patients.30–32 While some studies suggest that pregnancy itself may be a risk factor for retinopathy progression after adjusting for HbA1c levels, the common practice of instituting tight control at the onset of pregnancy confounds our ability to conclude that pregnancy itself is the cause of retinopathy acceleration.27
Hormonal theory
Beyond the rapid glycemic normalization in pregnancy, hormonal changes are suspected to exacerbate diabetic retinopathy. The characteristic progesterone surge of pregnancy may upregulate intraocular VEGF33 and result in increased retinal capillary leakage and neovascularization. Additionally, placental hormones create a physiologically adaptive insulin-resistant state to ensure an adequate supply of maternal glucose to the fetus to optimize intrauterine growth. A key factor is human placental growth hormone which appears to regulate the maternal levels of insulin-like growth factor-1 (IGF-1). Like human placental growth factor, IGF-1 increases after 20 weeks of pregnancy. Transgenic mice studies have demonstrated that human placental growth hormone can cause an insulinresistant state, although the precise mechanism is unknown.34 Additionally, increasing ser um IGF-1 levels may promote retinal neovascularization by supporting vascular endothelial growth factor induction of endothelial cell proliferation.35
Hemodynamic theory
Altered retinal hemodynamics may also play a role in retinopathy exacerbation. Pregnancy is noteworthy for extensive hemodynamic and cardiovascular changes. Blood volume increases on average about 45% above non-pregnancy levels, cardiac output is increased, and peripheral vascular resistance is decreased.34 This increased blood flow, coupled with an impaired retinal vascular autoregulatory response in diabetes, may result in a hyperdynamic retinal capillary blood flow that exacerbates diabetic retinopathy via increased sheer on the vascular endothelium and a resultant net increase in fluid leaving the capillaries.36 Some studies have demonstrated increased flow in the retinal circulation and hyperperfusion in all pregnant diabetic subjects compared with non-diabetic pregnant women,37 while others have shown increased retinal blood flow only in pregnant women with pre-existing36 or progressive diabetic retinopathy.38 Another small study showed a fall in retinal volumetric blood flow in pregnant diabetic subjects and a more profound decrease in retinal venous diameter in the diabetic subjects compared with non-diabetic pregnant controls.39 The contradictory results of these studies may be explained by different patient populations or differing methods of assessment of the retinal circulation.
Immunoinflammatory theory
Another theory involves proinflammatory factors. Diabetic retinopathy has been suggested to be a lowgrade inflammatory disease, with leukocyte adhesion to the retinal vasculature possibly resulting in retinal vascular dysfunction.40,41 During pregnancy, increased inflammation has been implicated in patients who develop gestational diabetes.42 A prospective study examining the relation of maternal cytokine levels with diabetic retinopathy showed that although the proinflammatory factors interleukin-6, c-reactive protein, and vascular cell adhesion molecule-1 were similar in the diabetic pregnant patients and non-diabetic controls, c-reactive protein levels were higher in pregnant women with retinopathy progression and worse glycemic control compared with their pregnant counterparts with stable retinopathy and tighter metabolic control. Another study examined glycodelin, an anti-inflammatory serum marker secreted from the endometrial glands during pregnancy, and found that low levels were associated with retinopathy progression in pregnant diabetic women.43
CLINICAL MANAGEMENT OF RETINOPATHY BEFORE AND DURING PREGNANCY
Preconceptional care is essential to reduce the risk of retinopathy progression during pregnancy. Both systemic and ocular care play an instrumental role.
Systemic management
Systemically, optimal metabolic control prior to pregnancy is essential. Rather than the aggressive tightening of glycemic control once the patient is pregnant, a gradual optimization of glycemic control prior to pregnancy avoids the rapid drop in HbA1c during pregnancy which has been associated with retinopathy progression.
Optimizing glycemic control prepregnancy is also essential for the health of the fetus. Multiple studies have demonstrated that the risk of fetal abnormalities increases with poor glycemic control.5,44–47 In a prospective cohort study of 301 Type 1 diabetic subjects with 573 pregnancies followed from 1985 to 2003,44 the prevalence of adverse outcomes varied six-old from 12% in the lowest HbA1c quintile to 79% in the highest quintile group. Specifically, when HbA1c levels were above 7%, there was an almost linear relationship between HbA1c level and risk of adverse fetal outcome. For every 1% increase in HbA1c level above 7%, the risk of adverse fetal outcome rose by 5.5%. Additionally, hypertension and elevated serum lipids should be controlled to reduce the risk of retinal changes. Such management not only reduces the risk of retinopathy progression, but is also important for the systemic health of the mother and fetus.
Ocular management and scheduling of dilated examinations
From an ocular perspective, all diabetic patients should have a dilated ophthalmic examination by an ophthalmologist or optometrist experienced in retinal evaluation prior to pregnancy and again during the first trimester of pregnancy. Depending on the individual patient’ s findings, additional imaging such as retinal photography, optical coherence tomography, and fluorescein angiography may be performed at the discretion of the examiner. The official guidelines for retinal care of pregnant women with Type 1 and Type 2 diabetes established by the American Academy of Ophthalmology are as follows:48
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree