Fig. 11.1
PML-RARα chimeric protein generated by t(15;17)(q22;q21) in APL. The chimeric PML-RARα protein retains critical domains of PML and RARα and promotes development of APL. PML-RARα fusion protein affects both nuclear receptor signaling and assembly of PML nuclear bodies
Table 11.1
Chromosomal translocations in APL and response to all-trans retinoic acid (ATRA)
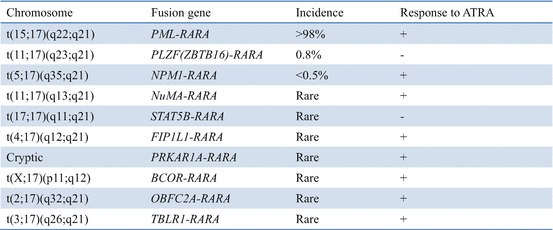
In addition to the presence of chromosomal translocation t(15;17) that produces PML-RARα, other genetic alterations have also been implicated in the development of APL [28, 29, 39, 40]. Expression of PML-RARA in mice is associated with long latency of onset of the disease and variable penetrance [41–44]. This indicates that secondary genetic abnormalities contribute to the pathogenesis of APL. A comprehensive mutational analysis of APL revealed that primary APL cells harbored FLT3-ITD (27%), FLT3 (16%), WT1 (14%), NRAS (10%), KRAS (4%), ARID1A (4.8%), and ARID1B (3%) mutations in 165 initial diagnosis samples [40]. These mutations, which cooperate with PML-RARα, appear to play a role in the pathogenesis of APL. On the other hand, the absence of mutations in other common AML-associated genes including DNMT3A, NPM1, TET2, ASXL1, and IDH1/2 was observed.
11.3 Molecular Mechanism of Action for ATRA
RARα is a member of the nuclear hormone receptor superfamily that acts as ligand-inducible transcriptional regulators [30, 31, 45]. RARα requires heterodimerization with retinoid X receptor α (RXRα) to bind the retinoic acid response element (RARE) located in the promoter regions of the target genes [31, 45]. Unliganded RARα can bind RARE and represses transcriptions through chromatin remodeling by recruiting transcription corepressors including mSin3A, NcoR, and SMRT [16, 46, 47]. These corepressors recruit histone deacetylase (HDAC) complex, resulting in histone deacetylation that leads to transcriptional silencing [16–18]. Retinoic acid (RA) binding to RARα induces dissociation of the HDAC complex and recruits coactivators such as p300/CBP, leading to histone acetylation and activation of transcription [18, 31]. One of the potential RA/RARα target genes is a transcription factor C/EBPε, which affects myeloid differentiation [48].
PML is the organizer of nuclear domains called PML nuclear bodies (NBs), which show a speckled pattern in the nucleus by PML antibody staining (Fig. 11.2) [30, 49–52]. Formation of PML NBs is associated with several stress responses, including telomere shortening, DNA damage, aggregation of misfolded proteins, deregulation of ribosome biogenesis, senescence, and proteasome inhibition [53]. NBs appear as domains assembled by oxidative stress and SUMO (small ubiquitin-like modifier)-ylation, whose assembly controls stress-induced posttranslational modification of various proteins. PML overexpression induces cell senescence, growth arrest, or apoptosis. In addition, PML NB formation is associated with self-renewal of normal or cancer stem cells [54].
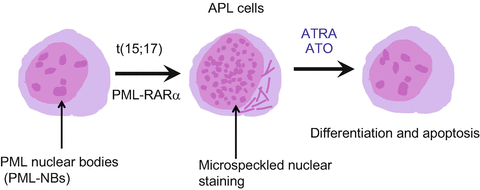

Fig. 11.2
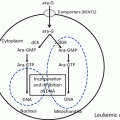
Schematic representation of the nuclear bodies and the effects of ATRA and arsenic trioxide (ATO). In normal myeloid cells, PML nuclear bodies (NBs) show a speckled pattern when stained by PML antibodies. In APL cells, PML-RARα disrupts PML NBs, leading to a microgranular pattern of NBs. Both ATRA and ATO elicit PML-RARα degradation, resulting in reformation of normal NBs and cell differentiation
APL is driven by a PML-RARα fusion protein that affects both nuclear receptor signaling and PML NB assembly [30–32, 51]. In APL cells, PML-RARα binds RARE through the DNA-binding domain of RARα in a dominant manner [30, 55]. PML-RARα mediates transcriptional repression of RARα target genes by recruiting corepressors, HDAC and DNA methyltransferase. Physiological dose of RA cannot dissociate the HDAC complex, leading to a maturational block in myeloid differentiation, which is thought to be an initial step of leukemogenesis [16–18]. In contrast, pharmacological doses of ATRA trigger dissociation of the HDAC complex and recruitment of coactivators [16–18, 56]. Thus, PML-RARα is a potent repressor of nuclear hormone receptor signaling, but it also disrupts PML NBs, resulting in a microgranulation pattern (Fig. 11.2) [30, 49–51]. PML-RARα can be SUMOylated at K160 residue of the PML protein to recruit death domain-associated protein (DAXX), which transcriptionally represses target genes [57].
ATRA and ATO target RARα and PML, respectively, the two distinct moieties of the disease-specific oncoprotein PML-RARα [30, 32]. Both ATRA and ATO elicit PML-RARα degradation, leading to reformation of normal NBs mediated by non-rearranged PML and cell differentiation (Fig. 11.2) [49–51, 56]. Arsenic-elicited PML degradation is initiated by SUMOylation of K160 [58]. ATO directly binds with PML at the C-C motif in the B2 domain, and PML SUMOylation can be induced by enhancing ubiquitin-conjugating enzyme 9 (UBC9) binding at the PML RING domain (Fig. 11.1) [59]. ATO induces SUMOylation of PML that recruits a SUMO-dependent ubiquitin ligase, RING finger protein 4 (RNF4), which enforces PML polyubiquitination [33, 34, 59]. PML is also required for some activities of p53. PML-RARα expression impedes p53 signaling and blocks the effects of p53 activation on cell death or clonogenic growth. Thus, NB disruption by PML-RARα impairs p53 key functions in controlling stemness or self-renewal, mediated by enforcing G0 arrest of stem cells, resulting in proliferation and aberrant stem cell self-renewal [58]. NB reformation in response to ATRA or ATO treatments precipitates apoptosis or senescence [60]. Moreover, ATRA induces degradation of PML-RARα and activates normal RARα and PML, resulting in differentiation of APL cells.
11.4 ATRA in the Treatment of Newly Diagnosed APL Patients
11.4.1 ATRA as a Differentiating Agent for APL
In the late 1980s, investigators in Shanghai demonstrated that the active form of retinoids, ATRA, could induce differentiation in APL cells, resolve life-threatening coagulopathy, and achieve a high CR rate [10]. Early studies of ATRA documented marked clinical efficacy in patients with both relapsed and newly diagnosed APL, indicating that ATRA has no cross-resistance against chemotherapeutic agents [61–63]. However, rapid increase of leukocytes is common, which is often accompanied by APL differentiation syndrome (DS; formerly RA or ATRA syndrome) [64]. Furthermore, most patients treated with ATRA alone after achieving CR suffer relapse [62]. Therefore, the combination of ATRA and cytotoxic chemotherapy has been incorporated in the frontline therapy for newly diagnosed APL.
11.4.2 ATRA in Induction Therapy
11.4.2.1 ATRA for Newly Diagnosed APL Patients in Multicenter Trials
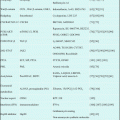
In the US intergroup study, previously untreated APL patients were randomly assigned to receive ATRA or chemotherapy which consisted of daunorubicin (DNR) plus cytarabine (Ara-C) during induction [65]. Although CR rates between the two groups were not different, 3-year disease-free survival (DFS) rates were 32% in the chemotherapy group (n = 174) and 67% in the ATRA group (n = 172) (P < 0.001). The 3-year overall survival (OS) rates were 50% for patients assigned to chemotherapy and 71% for patients who received induction with ATRA (P < 0.001). In European APL91 study, a multicenter randomized trial compared chemotherapy with DNR plus Ara-C and ATRA combined with the same chemotherapy during induction in newly diagnosed APL patients aged 65 years or younger [66]. The trial was terminated early after the first interim analysis, as 1-year event-free survival (EFS) was significantly higher in the ATRA group (79% vs. 50%, P = 0.001). These initial randomized studies indicate that ATRA should be incorporated in frontline therapy for newly diagnosed APL.
Thereafter, several multicenter studies conducted during the past decade have contributed to optimizing the antileukemic efficacy of ATRA when used in combination with chemotherapy for induction therapy (Table 11.2) [21, 65, 67–78]. In the GIMEMA and PETHEMA studies, simultaneous use of ATRA and idarubicin (IDR) (AIDA) during induction was highly effective and led to CR in 90–95% of patients; resistance to AIDA therapy was reported only infrequently [71–74]. In the Japan Adult Leukemia Study Group (JALSG) studies, ATRA was administered to all patients at a 45 mg/m2/day until CR or for 60 days [21, 24, 63, 77]. Addition of chemotherapy during induction depends on the initial WBC count and leukemic cell count in the peripheral blood (Fig. 11.3) [21]. Subsequent consolidation therapy consisted of three courses of intensive chemotherapy with anthracyclines and Ara-C. In the JALSG AML87 and AML89 studies, 6-year DFS rates in CR patients who were treated with chemotherapy alone were 40% and 45%, respectively [63]. In the JALSG APL92 and APL97 studies, which included ATRA plus chemotherapy during induction followed by consolidation chemotherapy, the 6-year DFS were 59% and 68.5%, respectively (Fig. 11.4) [24, 77, 78].
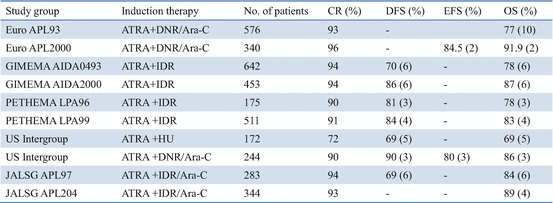
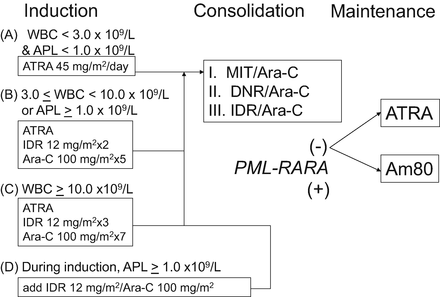
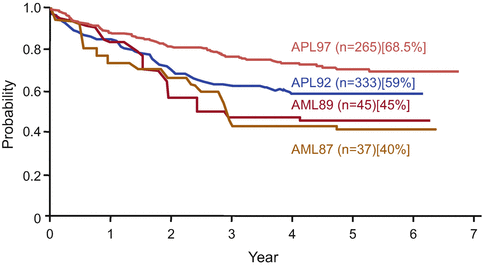
Table 11.2
ATRA and chemotherapy for newly diagnosed APL in multicenter trials


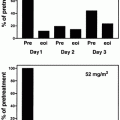
Fig. 11.3
Therapeutic protocol in the JALSG APL204 study. ATRA was administered to all patients at a daily dose of 45 mg/m2 until the patients achieved complete remission or for 60 days, whichever occurred first. Chemotherapy regimen depended on initial WBC count and APL cell count in the peripheral blood. For example, patients whose pretreatment WBC counts were 3.0–10.0 × 109/L and/or the APL cell count exceeded 1.0 × 109/L (group B) received idarubicin and cytarabine. Consolidation therapy consisted of three courses of intensive chemotherapy. After completion of the third consolidation course, patients in molecular remission of PML-RARA were then randomly assigned to either oral administration of ATRA (45 mg/m2/day) or to oral tamibarotene (Am80; 6 mg/m2/day), both for 14 days every 3 months. Maintenance therapy was continued for up to 2 years for a total of up to eight courses in both groups

Fig. 11.4
Disease-free survival in APL patients who achieved complete remission in the Japan Adult Leukemia Study Group (JALSG) APL studies. In the JALSG AML87 and AML89 studies, APL patients were treated with chemotherapy alone, whereas APL patients registered in the JALSG APL92 and APL97 studies were treated with ATRA and chemotherapy. In the APL97 study, chemotherapy regimens during induction and consolidation consisted of anthracyclines and cytarabine instead of anthracyclines and biphenyl cytarabine in the APL92 study
In current multicenter trials, more than 90% of newly diagnosed APL patients treated with ATRA plus chemotherapy achieve CR, of whom 20–30% subsequently relapse and then approximately 60–80% of patients have DFS (Table 11.2) [67–78]. Non-cross-resistance between ATRA and chemotherapeutic drugs appears to contribute to the significant improvement in EFS. However, several major clinical problems still account for treatment failure, including early death, death during consolidation, and disease relapse. Induction failure is mainly caused by hemorrhage, DS, or infection [3, 4, 23, 64]. Another sizable subset of patients dies in CR from complications of consolidation, mainly from infection due to chemotherapy-associated myelosuppression [67–78]. Optimal chemotherapy during induction and post-remission in these high-risk groups remains to be determined. Because of the excellent response with ATRA plus chemotherapy, hematopoietic stem cell transplantation (HSCT) is generally not indicated in first CR but might be considered for second CR patients [79, 80].
These multicenter studies led to a proposal to assess relapse risk in APL patients treated with ATRA and chemotherapy. The JALSG APL92 study revealed that initial WBC count at diagnosis can independently predict DFS [24]. The predicted 4-year DFS rate was 68% in patients whose initial WBC count was less than 10 × 109/L and 42% in those whose initial WBC count was >10 × 109/L (P = 0.0007). Relapse-risk groups proposed by the PETHEMA group were as follows: low risk, WBC count <10 × 109/L and platelet count ≥40 × 109/L; intermediate risk, WBC count <10 × 109/L and platelet count <40 × 109/L; and high risk, WBC count ≥10 × 109/L [81]. This so-called Sanz risk score, essentially based on WBC and platelet counts at diagnosis, allowed further refinement of frontline APL therapy through up-front risk assessment and use of risk-adapted ATRA and chemotherapy.
To decrease mortality in CR, the PETHEMA group reduced the intensity of consolidation by avoiding Ara-C in the LPA99 study (Table 11.2) [74]. They observed a lower rate of death in CR (2%) and a low cumulative incidence of relapse (CIR) (10%). On the other hand, the APL2000 trial conducted by the French-Belgian-Swiss APL group in patients with WBC count less than 10 × 109/L, which examined the role of Ara-C in addition to ATRA and anthracyclines, found a significantly higher 2-year CIR (15.9% in the no Ara-C group vs. 4.7% in the Ara-C group, P = 0.011) and lower 2-year OS (89.6% vs. 97.9%, P = 0.0066) in patients treated without Ara-C [69]. To assess these discrepancies between the LPA99 and APL2000 studies, particularly regarding the effect of Ara-C, these two studies were jointly analyzed [82]. In the low- and intermediate-risk groups, 3-year CIR was 14.3% and 4.2% (P = 0.03) in the APL2000 and LPA99 trials, respectively. OS were 95.6% and 93.8% (P = 0.53) in the APL2000 (n = 96) and LPA99 (n = 402) trials, respectively. In the high-risk group, 3-year CIR was 9.9% and 18.5% (P = 0.12), and 3-year OS was 91.5% and 80.8% (P = 0.026) in the APL2000 (n = 82) and LPA99 (n = 104) trials, respectively. These results suggest that ATRA combined with a high cumulative dose of IDR without Ara-C but with a maintenance treatment like the PETHEMA approach may give excellent results with limited toxicity in low- and intermediate-risk APL, whereas the addition of Ara-C to ATRA and anthracyclines in high-risk patients may result in a trend toward lower CIR and a better OS.
11.4.2.2 Hemorrhage
Recent registry studies show that hemorrhage is still a major problem, as many patients fail to be promptly diagnosed and/or receive appropriate treatment in time [83]. Remission induction failures are a major stumbling block even in current APL therapy. Although infection is the predominant cause of death in AML, hemorrhage is the most common cause of death during induction in APL patients, followed by DS and infection [23]. The majority of lethal hemorrhages occurred early during induction. Repletion of coagulation factors and platelet with blood products is the mainstay of supportive treatment in APL patients. In most multicenter studies, platelet transfusions are given to maintain a platelet count of at least 30 × 109/L until resolution of the coagulopathy [14]. Patients with active coagulopathy also receive fresh-frozen plasma, cryoprecipitate, or fibrinogen to maintain a fibrinogen level above 1.5 g/L. Nevertheless, fatal hemorrhagic events have been seen in 2–5% of patients treated with ATRA and chemotherapy [23]. APL cells mediate hemorrhagic diathesis through multiple mechanisms that lead to a combination of consumptive coagulopathy and primary hyperfibrinolysis. Exposure of tissue factor, annexin II, and microparticles from the leukemic cells has been implicated in the coagulopathy [3, 4]. Therefore, not only aggressive supportive care but also prompt treatment with ATRA (with or without ATO) is the most important step in preventing bleeding complications [14]. In a randomized comparison of ATRA plus chemotherapy vs. ATRA plus ATO, the ATRA and ATO combination resulted in decreased early death, which suggests that ATO exerts a better counteractive effect on coagulopathy compared with chemotherapy [84, 85].
In the JALSG APL97 study, 18 patients (6.5%) suffered severe hemorrhage and nine of them succumbed to early deaths [86]. Although most of them were receiving frequent transfusions, the targeted levels of platelet counts (30 × 109/L) and plasma fibrinogen (1.5 g/L) were reached at the day of bleeding in only 71% and 40%, respectively. Risk factor analysis identified three pretreatment variables associated with severe hemorrhage, initial fibrinogen level, WBC count, and performance status. In addition, patients with severe hemorrhage were more likely to develop DS or pneumonia than patients without hemorrhage. In the PETHEMA study, a multivariate analysis of pretreatment characteristics showed that abnormal creatinine level, high peripheral leukemic cells, and the presence of coagulopathy were associated with an increased risk of fatal hemorrhage [23].
11.4.2.3 Differentiation Syndrome (DS)
DS, formerly known as ATRA syndrome, is a life-threatening complication in patients with APL who undergo induction therapy with ATRA or arsenics [64, 87, 88]. The syndrome is clinically characterized by unexplained fever, weight gain, peripheral edema, dyspnea with interstitial pulmonary infiltrates, pleural or pericardial effusion, hypotension, and renal failure. The reported incidence of this syndrome ranges from 2% to 27% [67–78]. This wide range is probably because of the different criteria used to diagnose DS and differences in induction therapy and prophylaxis. Early addition of chemotherapy to ATRA and administration of high-dose dexamethasone at the onset of the first symptoms appeared to reduce the DS-related mortality to 1% or less in the recent trials. Although predictive factors of DS have not been determined, DS is associated with increased differentiated neutrophils that secrete inflammatory cytokines such as IL-6, IL-8, and TNFα. Prevention of hemorrhage and DS during induction is critical to achieve CR, because primary resistance to ATRA is an exception in newly diagnosed APL with t(15;17).
The Spanish PETHEMA group analyzed the incidence, characteristics, prognostic factors, and outcome in 739 patients treated with AIDA in the LPA96 and LPA99 studies (Table 11.2) [89]. Diagnosis of DS was made according to the presence of the following signs and symptoms described by Frankel et al. [64]: dyspnea, unexplained fever, weight gain greater than 5 kg, unexplained hypotension, acute renal failure, and particularly a chest radiograph demonstrating pulmonary infiltrates or pleuropericardial effusion. In this study, patients with four or more of the above signs or symptoms were classified as having severe DS, while those with two or three signs or symptoms were considered to have moderate DS. Of the 739 patients, 183 (24.8%) experienced DS including 93 (12.6%) with severe DS and 90 (12.2%) with moderate DS (Table 11.3). The most common manifestations of severe DS were dyspnea (95%), pulmonary infiltrates (81%), unexplained fever (74%), weight gain of more than 5 kg (68%), pleural effusion (58%), and renal failure (46%). DS occurred at a median of 12 days after starting ATRA treatment (range, 0–46 days). Severe DS occurred comparatively early, at a median of 6 days, while moderate DS appeared after a median of 15 days. The first peak occurred during the first week of ATRA treatment in 47% of patients with DS, and subsequently later cases of DS developed in the third week (25%), in the fourth week (19%), and after day 29 (3%). Development of moderate DS had no impact on the mortality during induction (6% with moderate DS vs. 7% without DS, P = 0.82), but severe DS was associated with an increased mortality (26%, P < 0.001) (Table 11.3). Notably, hemorrhagic mortality was also higher in patients who developed severe DS than in those with moderate DS or no DS (P = 0.02). In addition, severe DS was also significantly associated with a higher frequency of thrombosis. WBC counts greater than 5 × 109/L and serum creatinine levels greater than 1.4 mg/dL at diagnosis were identified as independent predictors of severe DS. Renal failure due to capillary leak syndrome caused by cytokine release in DS may explain the association between high serum creatinine and severe DS. Although the preemptive use of corticosteroids at the earliest clinical manifestations of DS has been adopted as the standard management, their prophylactic use is controversial [14]. A statistically significant reduction in the incidence of severe DS, but not in DS-related mortality, was probably associated with prophylactic use of prednisolone during an early 15-day interval in the LPA99 study [74]. As severe DS is associated with higher morbidity and mortality during induction, risk-adapted strategies that focus on patients with adverse risk factors deserve further investigation.
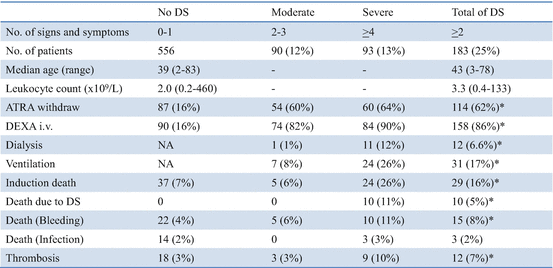
Table 11.3
Management and outcomes of APL differentiation syndrome (DS) in the PETHEMA LPA96 and LPA99 studies

11.4.3 ATRA in Consolidation Therapy
In the LPA99 trial, the Spanish PETHEMA group reported that a risk-adapted strategy combined with ATRA and anthracycline monochemotherapy for both induction and consolidation, followed by maintenance with ATRA and low-dose methotrexate (MTX) and mercaptopurine (6MP), resulted in a higher antileukemic efficacy than in the previous LPA96 trial (Table 11.4) [74]. Intermediate- and high-risk patients received ATRA (45 mg/m2/day for 15 days) combined with anthracycline for three courses during the consolidation phase in the LPA99 study. This risk-adapted strategy combining ATRA and anthracycline monotherapy for consolidation led to significantly improved 5-year CIR and DFS rates in the intermediate-/high-risk patients (Table 11.4).
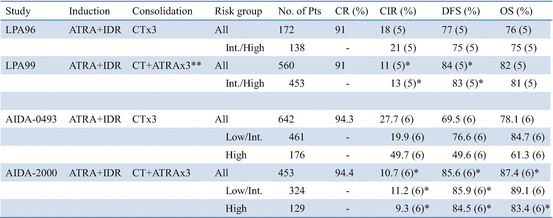
Table 11.4
ATRA in consolidation therapy

In the Italian GIMEMA Cooperative Group, the risk-adapted AIDA-2000 trial was designed to investigate the effects on patients’ outcomes of two main modifications from the original AIDA-0493 study: the omission of Ara-C in the low-/intermediate-risk group and the addition of ATRA (45 mg/m2/day for 15 days) during three courses of consolidation for all risk categories [72]. A total of 642 and 453 adult patients, aged 18–60 years, were enrolled in the AIDA-0493 and AIDA-2000 trials, respectively. The 6-year DFS rates were 69.5% in the AIDA-0493 and 85.6% in the AIDA-2000 (P < 0.0001) (Table 11.4). The 6-year CIR rates were 27.7% and 10.7% for all risk groups in the AIDA-0493 and AIDA-2000 studies, respectively (P < 0.0001). Percentages of CNS relapses were 2.5% and 2.1% in the two studies. This study shows that a risk-adapted post-remission strategy using variable chemotherapy intensity and addition of ATRA for consolidation resulted in significantly improved outcomes in patients with newly diagnosed APL. The benefit appears to result both from reduced toxicity and from increased antileukemic efficacy in the AIDA-2000 study. In the high-risk group, especially, addition of ATRA during each consolidation cycle significantly improved DFS and CIR rates in the AIDA-2000 compared with the AIDA-0493 study (Table 11.4). These Italian and Spanish studies document that the combination of ATRA plus chemotherapy in both induction and consolidation phases may contribute to improvement of CIR and DFS in high-risk group patients.
11.4.4 ATRA in Maintenance Therapy
The US intergroup study showed that maintenance treatment with continuous ATRA during 1 year significantly reduced relapses and improved survival (Table 11.5) [65]. On the other hand, the JALSG APL97 study showed that intensified maintenance with six courses of chemotherapy including biphenyl Ara-C and anthracyclines did not improve DFS in patients who achieved molecular remission after three courses of consolidation [77].
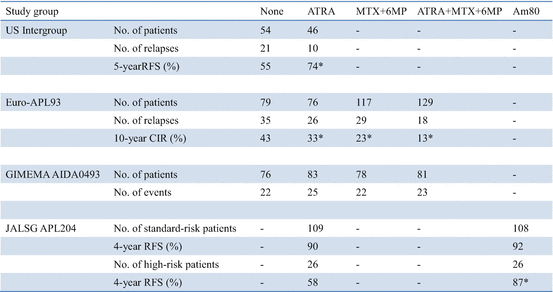
Table 11.5
ATRA in maintenance therapy

Of the 401 patients randomized for maintenance treatment on the European APL93 study, the 10-year CIR rates were 43.2%, 33%, 23.4%, and 13.4% in patients with no maintenance, maintenance with ATRA alone, maintenance with chemotherapy (6MP and MTX) alone, and both, respectively (P < 0.001) (Table 11.5) [67, 68]. Maintenance with 6MP and MTX significantly improved 10-year OS (85.2% vs. 79.2%; P = 0.02), but OS was not significantly modified by maintenance with ATRA (82.7% vs. 79.4%; P = 0.44). Among patients with WBC counts ≥5 × 109/L, the 10-year CIR rates were 68.4%, 53.1%, 32.8%, and 20.6% with no maintenance, ATRA alone, chemotherapy alone, and both, respectively (P < 0.001). In patients with WBC counts <5 × 109/L, the 10-year CIR rates were 29.2%, 22.9%, 21.0%, and 11.5% with no maintenance, ATRA alone, chemotherapy alone, and both, respectively (P = 0.069). The beneficial effect of maintenance with intermittent ATRA and continuous 6MP + MTX, with an additive effect of the two modalities, was particularly clear in patients with WBC counts higher than 5 × 109/L; the relapse rate dropped from 68.4% with no maintenance to 20.6% with combined maintenance.
An updated Italian GIMEMA group study, using the same randomization design as in the APL93 trial for maintenance (i.e., no maintenance vs. ATRA vs. 6MP + MTX vs. both), showed no difference in relapse among maintenance arms (Table 11.5) [71]. The benefit of maintenance may in fact depend on prior induction and consolidation therapy. For example, both GIMEMA0493 and JALSG APL97 studies used IDR as anthracycline for induction and consolidation chemotherapy, whereas the US and European studies, which showed a large benefit for maintenance, used DNR, which may be less effective than IDR for APL [65, 67, 71, 77]. In addition, it should be noted that in the GIMEMA and JALSG studies, only patients with molecular remission after three courses of consolidation were enrolled to the randomized study for maintenance, whereas patients with hematological remission after two courses of consolidation were randomized to maintenance in both the US and European studies. These conflicting evidences indicate that the relative benefit of maintenance depends on the prior induction and consolidation therapies. Therefore, it is appropriate to use maintenance in conjunction with treatment protocols in which it has been shown to confer benefit [14].
11.4.5 Long-Term Follow-Up for Newly Diagnosed APL
The European APL group reported very long-term outcomes of APL after treatment with ATRA and chemotherapy [68]. In the APL93 trial, 576 newly diagnosed APL patients were enrolled from 97 European centers. A total of 533 (92.5%) patients achieved CR, 42 (7.3%) had early deaths, and one (0.2%) had resistant leukemia. With a median follow-up of 121 months, 142 (26.6%) of the 533 patients who achieved CR had relapsed, 59 (11%) had died in CR, and 329 (61.7%) remained in first CR (Table 11.6). The 10-year CIRs were 16.5% in the 306 patients <65 years with WBC count <5 × 109/L, 37.9% in the high WBC count group, and 9.3% in the elderly group (P < 0.001). The estimated 10-year OS in the APL93 study was 77%. Most relapses occurred before the arsenic era, and 79% were treated with chemotherapy combined with ATRA. A total of 88% of the patients achieved second CR, and 63% of them subsequently underwent autologous or allogeneic HSCT. Of the 533 patients who achieved CR, 59 (11%) died in first CR, including 23 who died during consolidation, 10 during maintenance, and 26 after the end of maintenance treatment. Sepsis was the leading cause of death in CR (n = 23), followed by secondary tumors (n = 10). Among the 23 fatal infections, 17 occurred during consolidation, but six occurred during maintenance.
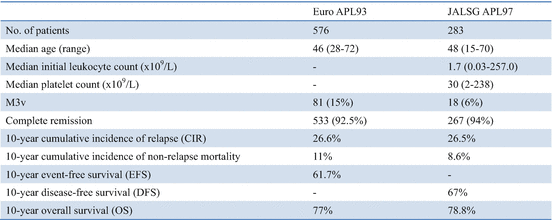
Table 11.6
Long-term follow-up for APL patients treated with ATRA and chemotherapy

The updated results of the JALSG APL97 study with a median follow-up of 7.7 years also confirmed favorable long-term antileukemic effects of ATRA combined with chemotherapy in newly diagnosed APL (Table 11.6) [78]. Interestingly, long-term observation in the European APL93 and JALSG APL97 studies showed similar results for CR rates, death in CR rates, 10-year CIR, and 10-year OS rates (Table 11.6). The major cause of death was sepsis secondary to myelosuppression, especially after consolidation courses and in elderly patients. Long-term outcome in the European APL93 and JALSG APL97 studies confirms that the combination of ATRA with chemotherapy can cure at least three-quarter of APL patients (Fig. 11.5). However, reduction of the incidence of non-relapse mortality (NRM) during consolidation in this highly curable disease is mandatory and requires reduction in the use of myelosuppressive drugs. In addition, the 10-year CIR was observed approximately one-quarter of APL patients treated with ATRA and chemotherapy (Table 11.6). Arsenic derivatives appear to have a growing role to play in these situations. Arsenic compounds are generally not myelosuppressive, and their use in first-line treatment of APL may reduce the total amount of chemotherapy and fatal infections [84, 85, 90, 91].
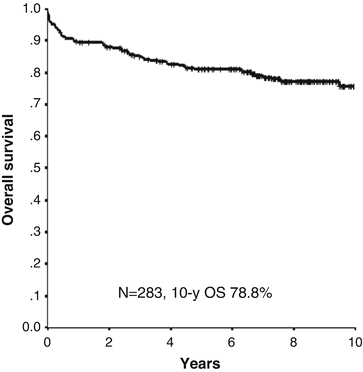

Fig. 11.5
10-year overall survival in patients with APL treated with ATRA and chemotherapy in the JALSG APL97 study. Of 283 patients, 267 (94%) achieved complete remission in the JALSG APL97 study. The 10-year cumulative incidences of non-relapse mortality and relapse were 8.6% and 26.5%, respectively. Estimated 10-year disease-free survival and overall survival rates were 67% and 78.8%
11.4.6 Unsolved Issues in APL Treated with ATRA and Chemotherapy
11.4.6.1 Relapse
In the European APL93 and JALSG APL97 studies, 10-year CIR rates were 26.6% and 26.5%, respectively (Table 11.6) [68, 78]. Relapse is still the main issue in APL patients treated with ATRA and chemotherapy, and drug resistance to ATRA is a critical problem. Relapse at extramedullary sites occurs up to 3–5% of APL patients treated with ATRA and chemotherapy [92, 93]. Extramedullary relapse such as the central nervous system (CNS) involvement can occur either in isolation or associated with marrow relapse. Management of relapse in the CNS and other extramedullary sites is a challenging issue. European LeukemiaNet recommends that CNS prophylaxis can be considered only for patients with hyperleukocytosis during induction [14]. Notably, first late relapse (>4 years) was also seen in about 3% of APL patients treated with ATRA and intensive chemotherapy [77, 94].
Inferior prognostic factor for relapse is high presenting WBC count (>10 × 109/L) in APL patients who received ATRA and chemotherapy [24, 81]. This high-risk group is also related to FLT3-ITD, PML break point (bcr3 isoform), and M3v [28, 29]. Expression of CD56 in APL cells has been associated with short DFS and extramedullary relapses [95–98]. A total of 72 (11%) of 651 patients were CD56+ (expression of CD56 in >20% APL cells) in the PETHEMA studies [97]. CD56 + APL was significantly associated with high WBC counts, bcr3 isoform, and extramedullary relapse. Moreover, 5-year CIR rates were 22% and 10% for CD56+ and CD56- APL patients (P = 0.006). In the JALSG APL97 study, expression of CD56 was found in 23 (9.6%) of 239 patients [98]. The CIR and EFS showed an inferior trend in CD56+ APL.
Detection of minimal residual disease (MRD) by RT-PCR or real-time quantitative PCR of PML-RARA in the marrow cells has suggested a benefit for preemptive therapy in patients who develop molecular relapse compared with treatment initiated at the time of hematological relapse [6, 99–102]. The risks of hemorrhagic early death and development of DS when patients present with overt disease argue strongly in favor of starting therapy as early as possible in cases of emergent relapse. This has provided the rationale for sequential MRD assessment by every 3 months during first 3 years after completion of consolidation therapy [14].
Several mechanisms of resistance to ATRA have been proposed, including the development of mutations in the ligand-binding domain (LBD) of the RARα, accelerated ATRA catabolism, and increased levels of cellular retinoic acid binding protein (CRABP), which induces retinoic acid metabolism [103, 104]. Genetic mutations that result in amino acid substitution in the RARα LBD are reported as underlying mechanisms in resistance to ATRA [105, 106]. In the RARA mutation, ATRA binding with LBD is generally impaired, and then ligand-dependent corepressor dissociation and degradation of PML-RARα by the proteasome pathway are inhibited. As a result, a maturational block in cell differentiation is observed in APL patients resistant to ATRA. Gallagher et al. [106] reported that LBD mutations were observed in 18 of 45 (40%) relapse patients treated with ATRA and chemotherapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree