Respiratory Physiology and Protection Against Hypoxia
Jeb S. Pickard
David P. Gradwell
There is something fascinating about science. One gets such a wholesale return of conjecture from such a trifling investment of fact.
—Mark Twain, Life on the Mississippi
RESPIRATORY PHYSIOLOGY
Respiration is the process by which an organism exchanges gases with the environment and, for most aerobic organisms, the critical portion of respiration consists of ensuring an adequate supply of oxygen. There is considerable geologic evidence that the Earth’s original atmosphere was anoxic, and it seems probable that life began under anaerobic conditions. Because habitable environments available to anaerobic organisms became relatively scarce due to the shift toward an oxidizing atmosphere, the development of enzyme systems capable of both utilizing and detoxifying oxygen was a practical necessity. This evolutionary step had an added ramification because the utilization of a reactive element such as oxygen unleashed a source of energy that allowed the development of ever more complex multicellular organisms. In turn, this required a more or less elaborate system capable of effective oxygen delivery.
The process of respiration is a simple one for unicellular organisms with gases exchanged through passive diffusion. Even in the most complex multicellular organisms, individual cells continue to passively exchange gases with their local environment in a similar manner. In complex organisms, however, each cell is surrounded by other cells competing for the same oxygen and eliminating carbon dioxide, which leads to several requirements. One requirement is an adequate source of oxygen, the definition of “adequate” depending in part on the metabolic rate, and another is an efficient delivery system. The poor solubility of oxygen in water impacts both requirements. For mammals, the source must be gaseous, with a sufficient pressure of oxygen. Gills are adequate to support poikilothermic organisms but, even in a tumbling mountain stream, the oxygen content of water is trivial compared to air. Also, for all but the simplest of multicellular organisms, the solubility of oxygen requires that the delivery system includes a carrier molecule.
In humans, the limbs of the oxygen delivery system consist of the following:
Ventilation—the process whereby pulmonary alveoli exchange gas with the atmosphere. Problems that may impact this part of the system include inadequate atmosphere, such as a hypoxic or hypercarbic environment, and airway obstruction.
Pulmonary diffusion—the process whereby gases are exchanged between the alveolus and the pulmonary capillary. The process of diffusion itself is simple and efficient, and clinical problems are rare. Most pulmonary problems that result in systemic hypoxia represent a failure to topographically match ventilation with perfusion.
Transportation—the shuttling of gases between lung and tissue, and back, through the vascular system. Clinical diseases related to oxygen transportation are common, and include anemia, hemorrhage, deficient cardiac output, and obstruction to blood flow.
Tissue diffusion—the exchange of gases between systemic capillaries and tissue cells. Again this is a simple process, although it can be impeded by increasing the distance between capillary and target cell, such as by tissueb edema.
Cellular utilization—the chemical reactions occurring within cells that employ oxygen. Compounds that interfere with these processes, such as cyanide, are toxic to most aerobic organisms.
Ventilation
Functional Anatomy of the Airways
In the normal human at rest, air is essentially completely humidified and warmed by the time it reaches the end of the trachea. From mainstem bronchi to the terminal bronchioles, the function of the airways is to conduct air to the respiratory zone. Because the airways from the nares to the terminal bronchioles do not participate in gas exchange, they constitute the anatomic dead space. In a normal adult male, this averages 150 mL at rest, although functional dead space may be increased by the addition of a rebreathing chamber, such as a mask. The resting bronchial tone found in normal airways serves to reduce anatomic dead space and thereby wasted ventilation, the trade-off being increased resistance to flow. Inflammation of the airways from a variety of causes commonly results in increased bronchial tone resulting in asthma, one of the most common diseases to afflict mankind.
Upon leaving the terminal bronchioles, gas flow enters the respiratory zone, comprising the respiratory bronchioles, alveolar ducts, and alveolar sacs. Although the total cross-sectional area of the airways increases as air flows peripherally, this increase is particularly dramatic as gas reaches the respiratory zone, and results in marked slowing of airflow velocity. Indeed, at this point the primary means of gas transport begins to be diffusion rather than convection, and gas movement is completely governed by diffusion at the level of the alveoli.
With levels of ventilation exceeding 8,000 L of air over a 24-hour period, the respiratory system is second only to the integument in exposure to the environment, and is necessarily far more delicate. Despite this constant assault, the parenchyma and distal airways are kept in a state of functional sterility by the mucociliary clearance system. The ciliated and secretory epithelial cells comprising this system constitute most of the cellls lining the conducting airways. Thin mucoid secretions float atop a watery sol layer, capturing particulate matter, and are wafted up the tracheobronchial tree to be passed through the glottis and swallowed. Although these secretions average approximately 100 mL/d, the normal individual is usually unaware of their production, a situation that changes when noxious stimuli such as infection, inflammation, or oxygen toxicity result in both impaired ciliary function and thickened secretions.
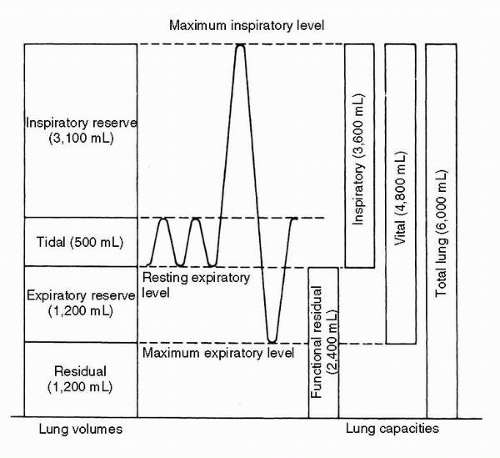
Lung Volumes
With each breath, the average adult at rest moves approximately 400 to 500 mL of air, an amount known as the tidal volume (TV). Inhalation is active, predominantly with the diaphragm, whereas exhalation is usually passive. The volume of air remaining in the lungs at the end of a passive exhalation is known as the functional residual capacity (FRC), and is determined purely by the balance between the elastic properties of the lung and the chest wall. If one exhales completely, the amount of air exhaled is designated as the expiratory reserve volume (ERV), and the remaining air that cannot be expelled (in the absence of a blow to the epigastrium) is known as the residual volume (RV). If instead one makes a maximal inspiratory effort from FRC, the volume inhaled is designated as the inspiratory capacity (IC), and the total volume of gas contained in the lungs is known as total lung capacity (TLC). The difference between TV and IC is designated the inspiratory reserve volume (IRV). The maximal amount of air that can be expelled from full chest expansion is the vital capacity (VC). These volumes and capacities are illustrated in Figure 2-1.
Average normal values for young adults are given in Table 2-1.
TABLE 2-1 | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
Volumes vary directlywith sitting height, explaining most differences between genders and races. Age tends to increase RV at the expense of VC. Note that because RV cannot be exhaled, neither RV nor any capacity that includes RV (TLC, FRC) can be measured by spirometry. Other techniques using gas equilibration or thoracic gas compression are required. The remaining volumes can be measured by a standard spirometer using a slow vital capacity maneuver. In modern clinical practice, spirometry has come to be synonymous with a forced vital capacity maneuver because this yields far more information about obstructive lung diseases, but an understanding of static lung volumes is fundamental to exploring the effects of pressure differentials and acceleration on the pulmonary parenchyma.
Inequality of Ventilation
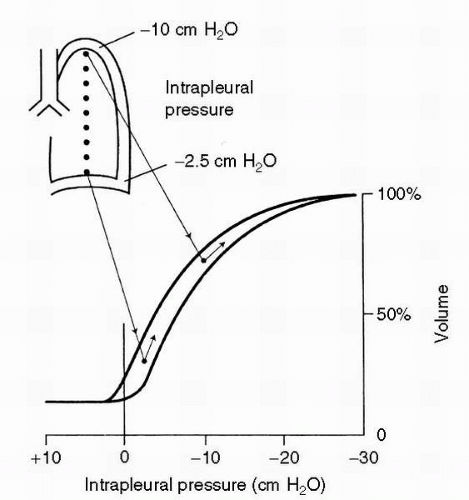
With the partial exception of the larger airways, the lung is anything but rigid, and while the chest wall and the elastic recoil properties of the lung largely determine overall lung volumes, gravity plays a profound role in the relative expansion of different portions of the lung. In the upright individual, with gravity influencing the lung inside the chest cavity, intrapleural pressure is more negative at the apex than at the base, and the alveoli in the upper portion of the lung are significantly more distended than are those at the base. In essence, the lung is trying to “settle” toward gravity, and although the entire lung cannot move inferiorly due to a relatively rigid chest wall, the parenchyma will shift until restrained by its own internal elasticity. Compared with alveolar units in the lung bases, apical alveoli are functioning over a flatter portion of the lung pressure-volume curve; as a result, they experience a smaller change in volume with any given breath, and hence receive less ventilation. This is illustrated in Figure 2-2. That these changes are due to gravity rather than anatomy is demonstrated by the fact that, in the supine individual, the posterior portions of the lung are better ventilated, although the changes are less marked because of the smaller distances involved.
The relative compression of the basal portions of the lung has other effects. As subjects exhale below FRC, small airways in the bases begin to close, trapping air inside distal alveoli. The volume at which this occurs, known as closing volume, is close to RV in healthy young individuals, but because of loss of lung elasticity over time, closing volume rises with age and may even approach FRC. This probably accounts for most of the decrease in resting arterial oxygen tension with advancing age. As one would predict, single-breath nitrogen washout testing during weightlessness (parabolic flight) has documented an absence of dependent airway closure with zero gravity (1).
Because these effects are dependent on gravity, it would follow that sustained accelerative forces (defined as multiples of the Earth’s gravity, or G) aggravate the topographic inequality in the lung. Dependent airway closure occurs routinely under G, and in combination with inequality of perfusion is responsible for a progressive decline in arterial oxygen levels with sustained acceleration. Ventilation with 100% oxygen partially offsets the hypoxia, but gives rise to a different problem, the syndrome of acceleration atelectasis (see Chapter 4). As noted earlier, airway closure in dependent lung parenchyma occurs before distal alveoli can collapse, which traps gas in those units. If 100% oxygen has been employed as the respirable gas mixture, the oxygen gradient from alveolus to pulmonary capillary will be such that the trapped gas is rapidly absorbed. (From the author’s personal observations of intact animal preparations, the speed at which this occurs is remarkable.) The resulting atelectasis may cause dyspnea, retrosternal discomfort, and coughing. The use of less than 70% oxygen has been shown to prevent this syndrome (2).
Perfusion
Although the intent is to follow the course of oxygen through the process of respiration, a slight diversion is necessary. It is not possible to discuss gas flow to the lungs without discussing pulmonary blood flow, particularly because most causes of deficient oxygenation result from a failure to match perfusion to ventilation.
Pulmonary Vasculature
Pulmonary arteries, unlike pulmonary veins, are intimately associated with their respective airways, branching with each generation of the airways. This presumably facilitates the process of hypoxic pulmonary vasoconstriction. In a manner analogous to the airways, the total cross-sectional area in the pulmonary arterial bed increases progressively in the smaller vessels; for instance, the total surface area of the pulmonary capillaries is 50 times that of the pulmonary arterioles. This results in considerable slowing of blood flow velocity, allowing ample time for gas exchange.
The pulmonary circulation is a low-pressure system, the vessels displaying compliance characteristics more nearly akin to systemic veins than to systemic arteries. Indeed, under the microscope, small pulmonary arteries are difficult to distinguish from small pulmonary veins. The result is that the entire vascular bed in the lungs participates in blood volume redistribution in response to hypo- or hypervolemic states, and in response to hydrostatic pressure changes. For example, performance of a Valsalva maneuver may force half of the volume from the pulmonary vascular bed.
Inequality of Perfusion
If the lung has a relatively low degree of structural integrity, blood has essentially none, and is profoundly influenced by gravity. In the systemic circulation, the effect of gravity is largely counterbalanced by a high-pressure system, with vessels capable of withstanding such pressures. In the pulmonary circulation, the thinner structure and distensibility of the pulmonary arteries result in regional distribution of blood flow which is markedly influenced by gravity. Additionally, with an average distance of less than 0.5 μm between capillary blood and the alveolar space, pulmonary capillaries are minimally supported by surrounding tissue. The result is that capillary blood flow is also affected by intra-alveolar pressures, as well as by the pressure drop from arterial to venous sides. The lack of tissue support also puts the pulmonary capillaries at risk. Animal studies have shown that at a capillary transmural pressure of 40 mm Hg, disruption of vascular integrity occurs (3). Similar damage has been documented in cases of high-altitude pulmonary edema, and is felt to be etiologic to the disorder, the damage perhaps brought on by nonuniform hypoxic pulmonary vasoconstriction (4).
Average pulmonary arterial pressures (systolic 25 mm Hg, diastolic 8 mm Hg, mean 15 mm Hg) are just sufficient to perfuse the lung apices in the upright human, but the amount of flow to the apical segments is low. Blood flow increases in a roughly linear manner as one proceeds from the apex to the base. With exercise, blood flow increases throughout the lung, and differences are less marked. Under conditions of weightlessness, there appears to be a marked reduction in regional inequality of blood flow; measurements of pulmonary diffusing capacity (DLCO) during a shuttle mission demonstrated dramatic improvement in DLCO, which did not appear to be explained by increased pulmonary capillary blood volume (5). (Despite the name, changes in diffusing capacity more often reflect variations in the amount of wasted ventilation than changes in the alveolocapillary membrane.) In contrast, during sustained acceleration the regional differences in perfusion become more profound. At +3 Gz (an inertial force of three times the Earth’s gravity, directed footward), the entire upper half of the lung is unperfused.
Another major determinant of regional inequality of pulmonary blood flow is hypoxic vasoconstriction. In contrast to systemic arteries, small pulmonary arteries constrict in response to hypoxia, but it is the intra-alveolar rather than the intraluminal oxygen tension that induces this response. Clearly a useful mechanism to blunt the hypoxemia that would otherwise occur with localized lung disease, hypoxic vasoconstriction occurs throughout the pulmonary vascular bed in response to environmental oxygen deficiency. In animal models, significant vasoconstriction has been shown to begin at an alveolar oxygen partial pressure (PAO2) of 70 mm Hg. In a healthy young adult, this would correspond to the expected PAO2 at an altitude of 2,438 m (8,000 ft). Species differences do exist, and it is not known whether significant hypoxic vasoconstriction begins in the human at the same altitude, but it is worth noting that this represents the altitude above which high-altitude pulmonary edema begins to appear.
Ventilation—Perfusion Matching
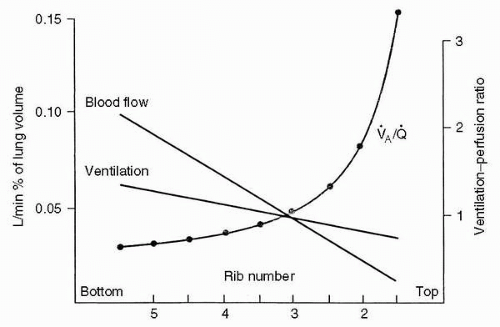
With the topographic distribution of airflow and blood flow noted earlier, it should be evident that most of the gas exchange in the resting upright individual occurs in the bases of the lungs. Because gravity has a greater effect on blood flow, the ratio of ventilation to perfusion ([V with dot above]/[Q with dot above] ratio) is maximal in the lung apices, and decreases as one proceeds to the bases. This is illustrated in Figure 2-3. The relative distributions of ventilation and perfusion result in a higher oxygen tension in the apical alveoli, which is thought to explain the proclivity of tuberculosis for the apices of the lungs.
Under normal conditions, the normal ventilation-perfusion inequalities merely result in a somewhat lower arterial oxygen partial pressure (PaO2) than might otherwise be expected. During sustained acceleration, the mismatch of ventilation and perfusion becomes highly significant. As gravity increases, the lung shifts caudally, stretching the apical alveoli and compressing those in the bases. At higher levels of+Gz, many of the basal alveoli never open at all. At the same time, pulmonary blood flow is almost entirely redirected to the bases. At high levels of G, physiologic shunting may account for half the pulmonary blood flow. The drop in PaO2 is affected both by the intensity of +Gz exposure, and by its duration. Even at +3 Gz, arterial blood gases do not reach a steady state after several minutes of exposure (see Chapter 4).
Pulmonary Diffusion
The area of the gas exchange surface in the lung is huge, from 50 to 100 m2, while the thickness of the membrane is usually under 0.5 μm. Diffusion is a passive process, determined in the case of a given gas by the difference in partial pressure across the membrane, by the diffusibility of the gas, and by the distance traversed. Diffusibility is a property of the gas itself, and is directly related to solubility, and inversely related to the square root of its molecular weight (see section Tissue Diffusion). Although carbon dioxide has a greater molecular weight than oxygen, it diffuses approximately 20 times as rapidly due to its much greater solubility. Pulmonary diffusion of carbon dioxide is not a limiting factor for gas exchange under any known circumstances. There are situations where oxygen diffusion limits pulmonary gas exchange, but in contrast to onceprevalent opinion, membrane thickness appears to rarely hamper oxygen diffusion even with diseased lungs. Under resting conditions oxygen equilibration between alveolar air and capillary blood is nearly complete before a third of the available time has elapsed. Therefore, there is enough of a reserve that equilibration can occur even when the membrane is thickened by interstitial disease, or when blood velocity increases with exercise. Diffusion limitation of oxygen may occur when both problems occur in combination, that is, exertion in the presence of interstitial lung disease. In the normal lung, diffusion limitation of oxygen only occurs in very hypoxic environments, particularly with exercise. At the summit of Mount Everest, for instance, diffusion limitation has been documented even at rest (6).
Gas Transport
Oxygen
Using Henry’s Law, one should be able to estimate the amount of gas contained in the given volume of blood based on the partial pressure of the gas in the equilibrium atmosphere, the solubility of the specific gas in the liquid, and the temperature. In fact, in the case of oxygen and carbon dioxide, blood contains far more gas than predicted, because of chemical reactions that occur with the blood components. The amount of oxygen that is actually dissolved in plasma under normal conditions is almost negligible with respect to metabolic needs. The solubility of oxygen in blood is 0.003 mL 02/100 mL blood/mm Hg, so that even at sea level with a PaO2 of 100 mm Hg, 100 mL of arterial blood contains only 0.3 mL of oxygen. Ventilation with 100% oxygen, which typically results in a PaO2 of approximately 650 mm Hg, will raise the level of dissolved oxygen to approximately 2 mL 02/100 mL blood, which represents only approximately 40% of the amount of oxygen consumed at rest. In fact, 100 mL of blood at a PaO2 of 100 mm Hg actually carries approximately 21 mL of oxygen, nearly all of it complexed to a carrier molecule, hemoglobin. (Because hemoglobin is nearly oxygen saturated at that oxygen tension, a higher concentration of oxygen results in a relatively minor increase in oxygen transport, representing that dissolved in plasma.)
Hemoglobin is a complex substance, composed of a tetramer of polypeptide chains, each chain surrounding a single heme moiety, which is a protoporphyrin ring complexed with ferrous iron. The polypeptide chains consist of two α-chains and two slightly longer non-α chains; in normal adult hemoglobin A, the latter are designated as β chains. Single amino acid substitutions on the globin chains can result in marked differences in stability and oxygen affinity; for instance, a substitution of valine for glutamic acid on the β chains results in sickle cell disease. Each heme moiety can bind a single oxygen molecule, so that each hemoglobin molecule can carry up to four oxygen molecules. Each gram of hemoglobin can combine with 1.39 mL of oxygen so that ignoring the plasma content of dissolved oxygen, the oxygen-carrying capacity of 100 mL of blood is 20.8 mL 02, given a normal complement of 15 g of hemoglobin.
The tetrameric arrangement of hemoglobin is crucial, allowing rapid uptake and efficient delivery of oxygen over a narrow range of tensions. Monomeric forms such as myoglobin have a high affinity for oxygen but are incapable of releasing it except at very low oxygen tensions. In a tetrameric arrangement, the monomers interact such that the binding
of the first oxygen molecule increases the affinity for oxygen of the remaining monomers. The physiologic result of this interaction is the sigmoidal oxygen dissociation curve seen in Figure 2-4.
of the first oxygen molecule increases the affinity for oxygen of the remaining monomers. The physiologic result of this interaction is the sigmoidal oxygen dissociation curve seen in Figure 2-4.
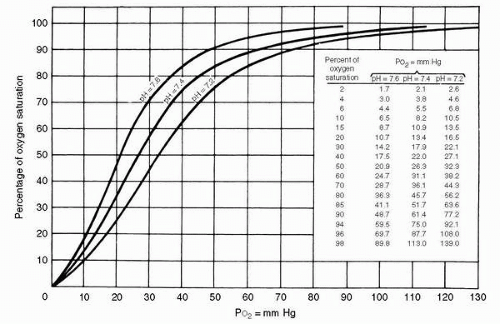
The shape of the curve is physiologically advantageous. In the relatively flat upper portion of the curve, a fall of 30 to 40 mm Hg in PaO2 from the “normal” level of 100 mm Hg, such as would occur at altitudes from 6,000 to 8,000 ft (1,829-2,438 m), results in only a 7% drop in arterial oxygen saturation. The curve accounts for some of the efficiency with which oxygen is loaded at the alveoli, because a large difference in partial pressure still exists when oxygen loading is nearly complete. The steepness of the lower portion of the curve means that a considerable amount of oxygen has been unloaded by the time the capillary oxygen tension has reached typical venous levels of 40 to 45 mm Hg. With a required oxygen tension at the mitochondrial level of 0.5 to 3 mm Hg, the large partial pressure difference between capillary and tissue facilitates diffusion into the tissues.
The actual position of the dissociation curve for normal hemoglobin A is influenced by temperature, by carbon dioxide tension, and by concentrations of hydrogen ion and intracellular 2,3-diphosphoglycerate; the last is a product of red cell metabolism, and increases in response to chronic hypoxia. A rise in any of these variables results in a shift of the curve to the right favoring oxygen unloading. The position of the dissociation curve is often expressed as the P50, the PO2 at which 50% of oxygen is saturated; the normal value is approximately 27 mm Hg. Most of the effect of carbon dioxide on the oxygen dissociation curve, known as the Bohr effect, is actually mediated through pH change, but while the other factors shift the curve in response to particular situations such as exercise or chronic hypoxia, the Bohr effect is dynamic. As CO2 is loaded at systemic capillaries the curve shifts rightward, resulting in further unloading of oxygen. Conversely, as CO2 is off-loaded at the pulmonary capillary, the curve shifts leftward, favoring oxygen uptake.
Anemia reduces the oxygen-carrying capacity of blood in proportion to the reduction of hemoglobin. However, loss of hemoglobin through oxidation of the ferrous iron to the ferric form (methemoglobin), or through complexing with carbon monoxide (carboxyhemoglobin), causes an impairment of oxygen transport disproportionate to the loss of available hemoglobin. In both cases, the dissociation curve for the remaining hemoglobin shifts leftward, impairing off-loading of oxygen from that hemoglobin which is still available for transportation.
In the resting adult, the systemic circulation off-loads approximately 5 mL O2 per 100 mL, resulting in a mixed venous PO2 of approximately 50 mm Hg and an oxygen saturation of 75%. With a system relying on passive diffusion there are limits to how much oxygen can be extracted, but a mixed venous saturation of 75% means a considerable amount of oxygen remains as a reserve. On the basis of an average resting cardiac output of 5 L/min, the total oxygen consumption calculates to be 250 mL O2/min in the 70-kg adult. Moderately active young men are able to increase their oxygen consumption to approximately 3 L/min (with up to 5 L/min in world class athletes), but cardiac output is incapable of matching this demand. With increased oxygen demand during maximal exercise, increased cardiac output accounts for approximately one third of the excess demand with the remainder being met by increased oxygen extraction from hemoglobin and a corresponding fall in mixed venous saturation.
The heart is in a unique situation regarding oxygen extraction. Even at rest, the myocardium removes approximately 12 mL oxygen per 100 mL blood. Therefore, blood
in the coronary sinus has a residual oxygen content of 8 mL per 100 mL blood, corresponding to a PO2 of 18 mm Hg. (Of course, the heart is never really at rest, which accounts for some of the difference compared with systemic venous blood.) Increased demand must be met by increased coronary blood flow, which is largely mediated through coronary vasodilation. In the left ventricle, increases in coronary perfusion are limited by the fact that flow can only occur during diastole, and exercise-induced tachycardia limits the available time. Undoubtedly, these limitations play a role in the cardiac reserve available for periods of exertion.
in the coronary sinus has a residual oxygen content of 8 mL per 100 mL blood, corresponding to a PO2 of 18 mm Hg. (Of course, the heart is never really at rest, which accounts for some of the difference compared with systemic venous blood.) Increased demand must be met by increased coronary blood flow, which is largely mediated through coronary vasodilation. In the left ventricle, increases in coronary perfusion are limited by the fact that flow can only occur during diastole, and exercise-induced tachycardia limits the available time. Undoubtedly, these limitations play a role in the cardiac reserve available for periods of exertion.
Carbon Dioxide
Because carbon dioxide is much more soluble than oxygen in blood, with a solubility of 0.0697 mL CO2/100 mL plasma/mm Hg, or approximately 24 times that of oxygen, dissolved carbon dioxide constitutes an appreciable percentage (approximately 10%) of the total elimination. Carbon dioxide, like oxygen, also undergoes chemical reaction with blood components, forming bicarbonate, which is responsible for approximately 60% of CO2 excretion, and combining with proteins to form carbamino compounds, which account for the remaining 30%.
Carbonic anhydrase catalyzes the hydration of CO2 to carbonic acid, which then readily forms hydrogen ion and bicarbonate. Most of the evolution of bicarbonate occurs within erythrocytes because carbonic anhydrase is absent from plasma. Bicarbonate moves into plasma by exchange with chloride. Within the erythrocyte, some of the hydrogen ions are bound to hemoglobin with the desaturated form proving to be the more efficient proton acceptor. Therefore, the off-loading of oxygen at the systemic capillary encourages the formation of bicarbonate, which reduces carbon dioxide tension, and increases its uptake. This mechanism is known as the Haldane effect. Desaturated hemoglobin also aids CO2 transport through another process. Globins are the most important substrate for the formation of carbamino compounds, and oxygen desaturation enhances this reaction, which again facilitates the further uptake of CO2 at the systemic capillary.
Nitrogen
Nitrogen is biologically inert, undergoing no chemical reaction with blood. Its concentration is determined by Henry’s Law, being directly proportional to the partial pressure of nitrogen in the adjacent gas. The actual content is also determined by the solubility of the gas, which in plasma at body temperature is 0.0088 mL N2/100 mL/mm Hg pN2, approximately three times that of oxygen. Nitrogen is five times more soluble in fat than in plasma, an observation that partially accounts for nitrogen acting as an anesthetic at high partial pressures, as demonstrated by the development of nitrogen narcosis during diving. The differential solubility also plays a role in decompression sickness (DCS) because different tissues with equivalent partial pressures of nitrogen contain different amounts of the gas (see Chapter 3).
Tissue Diffusion
There exist approximately 50 billion capillaries in the human body, with a combined cross-sectional area more than a thousand times the cross-sectional area of the aorta. This ensures a slow enough flow to allow adequate time for exchange of gases and nutrients. It is rare for a cell to be more than 30 to 50 μm from the nearest capillary. This distance is considerably greater than the 0.5 μm between the alveolus and the capillary, but the latter’s role is to provide gas exchange for the entire organism, and the pulmonary process must be more efficient.
The exchange of oxygen from capillaries to cellular mitochondria occurs along a gradient. Fick’s Law of diffusion describes the rate of transfer of a gas through a tissue as inversely proportional to thickness (T), and directly proportional to tissue area (A), partial pressure difference, and a constant, D.
Vgas = A/T × D(P1 − P2)
The value of the constant D is dependent upon the particular gas and tissue because it is directly proportional to the solubility of the gas in the tissue, and inversely proportional to the square root of the molecular weight of the gas.
To increase diffusion of oxygen into tissues, the systemic circulation can respond by increasing the flow through capillaries, which increases the partial pressure gradient (P1 − P2) for oxygen diffusion at the distal portion of the capillary. More importantly, it can also increase the number of open capillaries, which increases the area (A) for diffusion, and decreases the distance, or thickness (T), which the gas traverses. The recruitment or rarefaction mechanism seems to be constantly operative, with terminal arterioles fluctuating in their level of resistance as local tissue demands change. Decreasing oxygen tension, rising carbon dioxide tension, and falling pH all stimulate perfusion of local systemic capillary beds. There appear to be considerable differences between organs, both with respect to the potential recruitment of capillaries, and with respect to the major stimulus. The brain appears to be particularly sensitive to the tension of carbon dioxide, and there is relatively little difference in capillary flow between rest and exercise. Coronary blood flow to cardiac muscle appears to be most sensitive to oxygen tension, but capillary recruitment is limited by the diastolic interval, as noted earlier. In the renal and splanchnic circulations, flow decreases in response to exercise, as it does in muscle groups that are not involved with exertion. In actively exerting muscle groups, local endothelial control in response to oxygen and carbon dioxide tensions and to pH overrides sympathetic stimulation, resulting in striking recruitment of capillaries and increased local circulation.
Cellular Utilization
Approximately 95% of oxygen consumption by individual mammalian cells involves direct oxidation of substrates by the mitochondrial cytochrome system, in the process known as oxidative phosphorylation. The oxidative process is vital for the production of energy. Production of adenosine
triphosphate (ATP), the common coin of intracellular energy transactions, is possible in the absence of oxygen, but the initial step in carbohydrate metabolism, the cytosolic conversion of glucose to pyruvic acid through the glycolytic pathway, is inefficient, releasing only two molecules of ATP for each molecule of glucose. In the absence of oxygen, pyruvic acid is converted into lactic acid, which as a waste product is far more difficult to excrete than carbon dioxide. Furthermore, the process is incapable of catabolizing fatty or amino acids. The uptake of pyruvic acid into the mitochondrion, with its ensuing catabolism through the Kreb’s cycle and oxidative phosphorylation, yields an additional 36 molecules of ATP, with the production of carbon dioxide and water. The former is excreted rapidly, and the latter is commonly beneficial; in certain desert animals the metabolic production of water functions as a significant source of hydration. The aerobic catabolic pathway is illustrated in Figure 2-5.
triphosphate (ATP), the common coin of intracellular energy transactions, is possible in the absence of oxygen, but the initial step in carbohydrate metabolism, the cytosolic conversion of glucose to pyruvic acid through the glycolytic pathway, is inefficient, releasing only two molecules of ATP for each molecule of glucose. In the absence of oxygen, pyruvic acid is converted into lactic acid, which as a waste product is far more difficult to excrete than carbon dioxide. Furthermore, the process is incapable of catabolizing fatty or amino acids. The uptake of pyruvic acid into the mitochondrion, with its ensuing catabolism through the Kreb’s cycle and oxidative phosphorylation, yields an additional 36 molecules of ATP, with the production of carbon dioxide and water. The former is excreted rapidly, and the latter is commonly beneficial; in certain desert animals the metabolic production of water functions as a significant source of hydration. The aerobic catabolic pathway is illustrated in Figure 2-5.
The electron transport system involved in the reduction of oxygen is tightly linked so that free radicals are not usually released into the cytosol. The intracellular oxygen tension required by the mitochondrion is only approximately 3 mm Hg, and values above this do not affect the rate of oxygen uptake. Additional energy requirements are met not by increased mitochondrial activity, but rather by additional numbers ofmitochondria. A small lymphocyte will have only a few mitochondria, whereas a hepatocyte typically possesses approximately a thousand. Curiously, the cell responsible for the transportation of oxygen is the cell with the least use for it, because erythrocytes lose their mitochondria as well as their nuclei as they mature; erythrocytes are obligate anaerobes, utilizing glycolysis to meet their minor metabolic needs.
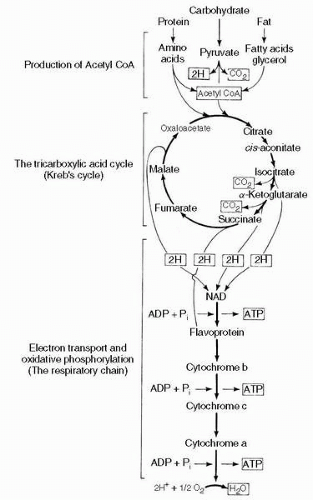 FIGURE 2-5 Cellular respiration. ADP, adenosine diphosphate; ATP, adenosine triphosphate. |
During oxidative catabolism, the ratio of carbon dioxide released to oxygen consumed is known as the respiratory exchange ratio, or respiratory quotient (RQ, or simply R). Catabolism of carbohydrates results in an RQ of 1.0; fatty acids and amino acids release less carbon dioxide relative to oxygen consumption, and have an RQ of approximately 0.7. Because diets are generally a mixture of all three components, the RQ usually averages between 0.80 and 0.85.
Control of Ventilation
Ventilatory control is maintained through a feedback control mechanism comprising a controller, the central nervous system (CNS); effectors, the muscles of respiration; and a number of sensors. The mechanism is linked between sensors and controller by afferent neurons and between controller and effectors by efferent neurons. Although the primary controller is located in the brainstem, it can be at least partially overridden by instructions from the cerebral cortex. The sensors are designed to detect changes in blood chemistry (chemoreceptors), or physical distortion of the lungs or chest wall (mechanoreceptors).
Cardiac rhythm is determined by a group of pacemaker cells whose unstable transmembrane potential results in spontaneous electrical activity, but if such a focus exists in the ventilatory controller it has never been identified. The rhythmic pattern of ventilation appears to depend on interconnected neurons in the medulla and pons; the actual generation and maintenance of rhythmic inspiration is likely determined by reciprocal impulses between these centers. Medullary centers consist of the dorsal respiratory group, which is active during inspiration, and the ventral respiratory group, different portions of which are active during inspiration and expiration. In addition, the ventral group contains neurons innervating upper airway muscles and bronchial smooth muscles. The pontine apneustic and pneumotaxic centers appear to influence respiratory timing. Experimental damage to the pneumotaxic center, in combination with vagotomy, induces an apneustic breathing pattern (prolonged inspiration), which disappears with wakefulness, and returns with sleep.
Ventilatory control is complex, and is affected by a number of inputs. Coordination of the respiratory muscles is required for the primary task of maintaining respiratory homeostasis and activities such as phonation, defecation, and parturition to name a few. While these activities are controlled by the cortex and brainstem, the intrinsic ventilatory rate is influenced most significantly by input from chemoreceptors. Under normal conditions, carbon dioxide tension is the primary driver for changes in ventilation. Changes in arterial carbon dioxide tension (PaCO2) are
sensed predominantly by central chemoreceptors, with a small input from peripheral chemoreceptors. The location of the central chemoreceptors is unclear although they appear to be distinct from the respiratory centers described earlier. Stimulation of the central chemoreceptors appears to be largely mediated by a decrease in the pH of brain interstitial fluid. The process is a smooth one; increases in PaCO2 induce a linear increase in minute ventilation, first by increasing the depth and then the rate of ventilation. Decreases in PaCO2 depress ventilation; one can induce apnea in the sleeping or anesthetized individual through artificial hyperventilation.
sensed predominantly by central chemoreceptors, with a small input from peripheral chemoreceptors. The location of the central chemoreceptors is unclear although they appear to be distinct from the respiratory centers described earlier. Stimulation of the central chemoreceptors appears to be largely mediated by a decrease in the pH of brain interstitial fluid. The process is a smooth one; increases in PaCO2 induce a linear increase in minute ventilation, first by increasing the depth and then the rate of ventilation. Decreases in PaCO2 depress ventilation; one can induce apnea in the sleeping or anesthetized individual through artificial hyperventilation.
It may seem counterintuitive that normal chemoreceptor input is based on carbon dioxide tension rather than oxygen tension but it is actually reasonable. Ventilation plays a minute-to-minute role in maintaining acid-base balance, whereas in healthy individuals the oxyhemoglobin dissociation curve ensures adequate arterial oxygen saturation despite such respiratory variation. This situation pertains unless oxygen tension reaches 60 mm Hg. From that point there is a rapid increase in minute ventilation in response to further reductions in PaO2. Therefore, unlike the linear increase in ventilation with rising PaCO2, there is a hyperbolic increase in ventilation with a falling PaO2. The response is affected by PaCO2, with a greater response to hypoxemia under hypercapnic conditions (7). The effect of different carbon dioxide tension on the hypoxic ventilatory response is displayed Figure 2-6.
The input from peripheral chemoreceptors is well illustrated by previously normal patients presenting with pneumonia. Those with milder cases and room air oxygen tensions reduced to 65-85 mm Hg will typically have near-normal pH and PaCO2 values. However, if the ventilation-perfusion mismatching is severe enough that the eupenic PaO2 would be reduced below about 60 mm Hg, then the usual pattern seen on arterial blood gases is a PaO2 maintained (if possible) near 60 mm Hg, with hyperventilation and respiratory alkalosis. Administration of supplemental oxygen typically raises the PaO2 and allows the return of PaCO2 and pH to more normal values.
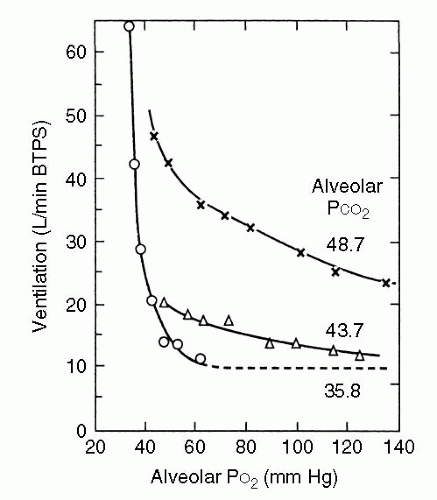 FIGURE 2-6 Hypoxic response curves. Note that when the PCO2 is 35.8 mm Hg, almost no increase in ventilation occurs until the PO2 is reduced to approximately 50 mm Hg. BTPS, body temperature and pressure, saturated with water vapor. (Modified by West JB Respiratory Phisiology, 2nd ed. Lippincott Williams & Wilkins, 1979, with permission; From Loeschke HH, Gertz KH. Einfluss des O2-Druckes in der Einatmungsluft auf die Atemtätigkeit der Menschen, geprüft unter Konstanthaltung des alveolaren CO2-Druckes. Pflugers Arch Ges Physiol 1958;267:460-477.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|