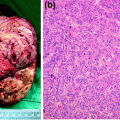
Fig. 11.1
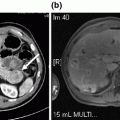
Computed tomography scan revealed the presence of right renal cell carcinoma with tumor thrombus extending into the retrohepatic inferior vena cava and right hepatic vein. a Coronal view showing the tumor thrombus extending into the retrohepatic inferior vena cava and right hepatic vein with liver congestion. b–d Transversal views showing the right renal cell carcinoma with tumor thrombus in the right renal vein extending into the inferior vena cava
Clinical Presentation
A more liberal use of imaging techniques is associated with increased incidental detection of asymptomatic renal cell carcinoma (RCC). But more than 90% of patients with tumor thrombus extending into inferior vena cava (IVC) present with symptoms [1]. The symptoms of patients with RCC extending into the IVC include hematuria (35%), abdominal pain (17%), and abdominal mass (2%) [2]. Other symptoms such as lower extremity edema, right varicocele, dilated superficial abdominal veins (caput medusae), or diagnosis such as pulmonary embolism, can reveal the diagnosis of RCC with caval tumor thrombus. When the tumor thrombus obstructs the hepatic veins, this may lead to abdominal pain, hepatomegaly, and ascites (Budd-Chiari syndrome) [3]. Also, paraneoplastic syndromes including hypertension, non-metastatic hepatic dysfunction (Stauffer’s syndrome), polycythemia, and hypercalcemia may have been observed in these patients [4].
Diagnosis and Assessment
RCC is the third most frequent genitourinary cancer and its prevalence is estimated to be between 2 and 3% of all malignant tumors in adults [5]. Due to its particular tropism for the venous system, there is a potential for extension into the renal vein, IVC (4–10%) and the right atrium (1%) [6, 7].
Assessment should be initiated with ultrasonography and MDCT, which are the primary methods for diagnosis of RCC with tumor thrombus. These two imaging techniques have demonstrated good specificity in detecting the presence of tumor thrombus, with a sensitivity of 65–90%, reaching 87% when used in combination.
Doppler ultrasound provides an estimate of the direction and speed of the blood flow within the IVC. However, the infrarenal portion of the IVC is imperfectly visualized in obese patients and when there is some gas interposition. Also, ultrasonography does not allow performing vessel reconstruction. MDCT is usually required for diagnosis of RCC, staging of IVC tumor thrombus, and surgical strategy [8]. It allows simultaneous thoracic screening. Magnetic resonance imaging (MRI) is usually considered as the gold standard for thrombus evaluation [9]. Fluorine 18-fluorodeoxyglucose (18F-FDG) PET-CT is commonly used in cancer staging disease. It can also be used to detect avid fluorodeoxyglucose thrombus, reflecting malignant thrombus. In our case, the patient demonstrates common presentation of right RCC with IVC tumor thrombosis.
Chronic obstruction of IVC by a tumor thrombus may lead to collateral vein development through deep and superficial venous collateral vessels. Four major collateral pathways have been described [10]: (i) The deep pathway, the most common, concerns the ascending lumbar veins, anastomosing with the azygos vein on the right side and the hemiazygos vein on the left side. Blood flow can also join vertebral, paraspinal, and extravertebral plexus. (ii) In the intermediate pathway, blood flow returns through the periureteric plexus bilaterally and the left gonadal vein to the left renal vein. (iii) The superficial pathway is constituted with the inferior epigastric and the abdominal wall veins, anastomosing with the superior epigastric veins and internal mammary veins to join the subclavians veins and the superior vena cava. (iv) The portal pathway concerns blood arising from lower extremities through the internal iliac veins to the hemorrhoidal plexus to join the inferior mesenteric vein and the portal system. The extent of development of these collateral veins may help in the decision whether to proceed or not to IVC reconstruction.
Staging of Intracaval Extension
The classification proposed by Neves and Zincke (i.e., Mayo Classification) [11] is the most widely used classification staging system of intracaval extension. This latter describes four levels of IVC extension: level I when extension only concerns the renal vein and/or the IVC < 2 cm; level II corresponds to extension within the IVC > 2 cm below the hepatic veins; level III corresponds to retrohepatic IVC and/or hepatic veins involvement; and level IV corresponds to extension above the diaphragm with or without atrial thrombus. The anatomic level of the tumor thrombus within the IVC dictates surgical strategy.
Surgical Strategy
This complex surgery requires a multidisciplinary management including experienced anesthesiologists and liver surgeons. This also requires an accurate preoperative imaging assessment by experienced radiologists.
In our patient, the level and extent of tumor thrombus was established preoperatively with MDCT and MRI. The patient had a tumor originated from the right kidney with an IVC tumor thrombus involving more than half of the circumference of the IVC, and extending into the retrohepatic IVC and the ostia of the right hepatic vein. There was no lymph node involvement or metastasis upon preoperative imaging. The relationship of the tumor thrombus to the liver, hepatic veins, diaphragm, and right atrium determined its staging as a level III tumor, according to the classification by Neves and Zincke.
For this case, we discussed three potential scenarios according to the preoperative clinical and radiological findings: (i) to perform right nephrectomy and IVC thrombectomy; (ii) to perform right nephrectomy and IVC resection; or (iii) to perform right nephrectomy, right hepatectomy and IVC resection. In all cases, it is necessary to perform the surgery under standard total vascular exclusion (TVE) of the liver. In case of hemodynamics instability at the moment of TVE, a venovenous bypass would be installed to maintain hemodynamics and prevent kidney and splanchnic venous congestion (see below) [12, 13]. This case of level III thrombus (extension to the right hepatic vein) did not theoretically require a combined abdominal-thoracic and sternotomy approach with a cardiopulmonary bypass. In addition, if TVE was predicted to last potentially longer than 60 min, the patient would have TVE of the liver with in situ hypothermic portal perfusion and venovenous bypass (usually cavo-porto-jugular, see below).
Intraoperative anesthesia was specifically adapted to the risks of massive bleeding, general hypothermia, rapid hemodynamic changes, and coagulation disorders subsequent to ischemia-reperfusion injury. Intraoperative monitoring and management included the following modifications in addition to standard noninvasive techniques: (1) two large-bore intravenous cannulas or a large-bore central catheter (a cordis with a triple-lumen central catheter); (2) an arterial catheter; (3) a Swann-Ganz catheter; (4) a rapid infusion device; (5) body and fluids warmers. Transesophageal echocardiography was used to provide real-time staging and surveillance of the cranial part and mobility of the thrombus.
Technical Aspects
Surgical Incisions
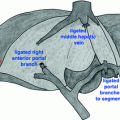
Surgical incision should be performed according to the anticipated level of tumor thrombus and the type of vascular control. A large number of different incisions are possible for the treatment of right renal tumors with retrohepatic IVC thrombosis. For our case, a transabdominal approach (J-shaped or bi-subcostal incision) or a thoracophrenolaparotomy using an oblique incision along the eighth or ninth intercostal space may be performed. The goal of these incisions was to facilitate proximal control of the suprahepatic IVC. This latter might be achieved either via an abdominal approach with or without pericardial incision, a transdiaphragmatic extrapericardial approach [14], or by sternotomy. In our patient, we performed a J-shaped incision with an intrapericardial approach of the IVC (Fig. 11.2).
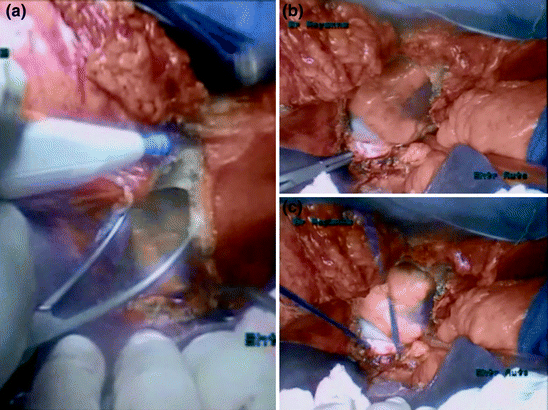

Fig. 11.2
Control of the suprahepatic inferior vena cava. a Pericardiotomy. b, c Control of intrapericardiac inferior vena cava
Surgery of the IVC and Hepatic Veins
The type of IVC surgery varied according to the location and the extent of the tumor thrombus, which was decided during surgery. If less than 30% of the circumference of the IVC wall was involved, it was sutured longitudinally. If wall involvement was between 30 and 50%, the IVC was sutured transversally to prevent stenosis of the vein. If the circumference of the wall was involved, the IVC was resected and replaced by a 20-mm-diameter external ring-reinforced PTFE.
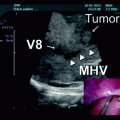
In our patient, intraoperative ultrasonography was first performed to confirm the presence of tumor thrombus in the retrohepatic IVC extending into the right hepatic vein and to rule out for any occult liver metastases. The surgical treatment of this patient required at least a radical right nephrectomy with en bloc resection of the retrohepatic IVC. It was not possible in our case to preserve the IVC because the thrombus was adherent to the caval wall and completely obstructed the IVC lumen.
Vascular Control of the IVC
The type of vascular control was planned following preoperative morphologic analysis. It was then adapted during surgery according to the intraoperative ultrasonography’s findings, with the aim of (1) minimizing the need for transfusion; (2) shortening ischemia time as much as possible; (3) maintaining stable systemic hemodynamics; and (4) to improve the tolerance of the remnant liver to ischemia-reperfusion injury in case of liver resection.
Two different vascular control techniques were possible: standard vascular exclusion of the liver, or two-step vascular exclusion of the liver. The standard TVE involved mobilization of the liver, and isolation of the suprahepatic and infrahepatic vena cava and the hepatic pedicle. The infrahepatic vena cava, hepatic pedicle, and suprahepatic vena cava were serially clamped following systematic ligation and division of the adrenal vein. After specimen removal, circulation was restored by unclamping successively the suprahepatic vena cava, the infrahepatic vena cava, and the portal triad. In the two-step TVE technique, TVE was performed, leaving a sufficiently long IVC stump below the confluence of the hepatic veins for replacement of the suprahepatic caval clamp by another clamp on the replaced retrohepatic vena cava, below the confluence of the hepatic veins (seen in Fig. 11.1c). En bloc resection of the specimen and of a segment of the vena cava could then be completed, with revascularization of the liver.
In our patient, we decided to control the vena cava in the pericardium (Fig. 11.2) for the following two reasons: (i) a safer control of the suprahepatic vena cava and (ii) to ensure that a sufficiently long stump of suprahepatic vena cava was available for secondary IVC reconstruction. The first strategic surgical step was to prepare the standard TVE by controlling the vena caval portion below (infrahepatic/renal IVC) and above the thrombus (supradiaphragmatic/intrapericardial IVC), particularly to avoid an embolism during preparation of the tumor-bearing kidney.
After complete mobilization of the right colon, liver, and a Kocher Maneuver, the right kidney and infrahepatic/infrarenal IVC were fully exposed. Kidney mobilization and control of the right renal artery was performed as usual (through either an anterior or posterior approach). The infrahepatic segment was dissected and encircled with a tourniquet. Left renal and gonadal veins were controlled and clamped before opening the IVC. The posterior surface of the infrahepatic/infrarenal IVC needs to be dissected carefully from the posterior abdominal wall by ligating and dividing all the lumbar veins found at this level, thus allowing complete circumferential control of this segment. The retrohepatic/suprahepatic infradiaphragmatic IVC segment should be circumferentially controlled. Exposure of this segment requires full liver mobilization. Then, the supradiaphragmatic IVC segment was controlled by opening the central tendon of the diaphragm. The pericardium was then opened so that the intrapericardial IVC can be encircled and taped below the confluence into the right atrium (Fig. 11.2).
Vascular exclusion of the IVC was then achieved (superior and inferior to the thrombus and the left renal vein). An opening to the lesser omentum allowed control of the hepatic pedicle with a tourniquet and vascular exclusion of the liver was also achieved (Fig. 11.3).
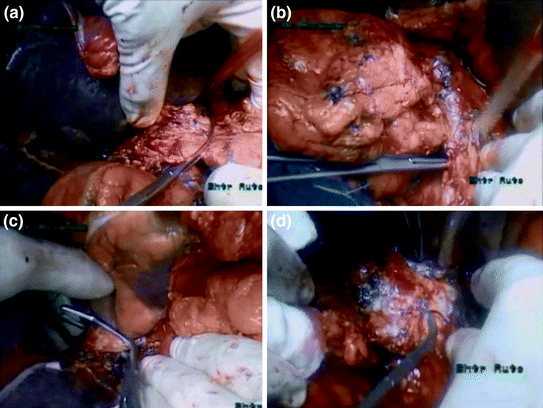

Fig. 11.3
Vascular control of the inferior vena cava. a Clamping of the portal triad. b Control of the infrahepatic/infrarenal inferior vena cava. c Clamping of the intrapericardiac inferior vena cava. d Control of the left renal vein
Adjunct Procedures: The Venovenous Bypass and Hypothermic Perfusion Techniques [12–14]
Caval occlusion at the suprahepatic or intrapericardial segments can compromise venous return to the heart in cases of partially occluding tumor thrombi, which results in decreased cardiac output, hemodynamic instability, and hypoperfusion. Extracorporeal circulation (i.e., venovenous or cardiopulmonary bypass) is indicated when resection followed by complex reconstruction of the inferior vena cava is performed (see second case presentation) or if caval-cross clamping is not hemodynamically tolerated despite adequate fluid loading (if cardiac output fell by more than 50% or a decrease in mean arterial pressure > 30%). The conventional technique for establishing vascular access for bypass involves cannulation of the portal (or inferior mesenteric vein) and right femoral veins to provide pump inflow and cannulation of the left axillary vein to accept pump outflow. This procedure implies a surgical dissection of the inferior mesenteric or portal veins that can be technically demanding in case of portal cavernoma, can prolong operating time, and can be associated with significant complications such as hematoma or bleeding. The puncture and cannulation of femoral and left axillary vein is then done under ultrasonography control as described by Oken et al. in 1994 [15].
If TVE was predicted to last potentially longer than 60 min, we advocate the use of hypothermia technique as an adjunct to increase the tolerance of the liver to prolonged ischemia. It has been demonstrated that every 10 °C fall in temperature of liver parenchyma decreases the liver enzyme activity by 1.5- to 2-fold. The principle of hypothermia approach is to perfuse the liver with conservation liquid used in organ transplantation and refrigerated at 4 °C. The temperature of the liver decreased then to 20 °C. The most popular methods of cooling for liver surgery include hypothermia portal perfusion and topical cooling (see second case presentation).
In our patient, we used neither venovenous bypass nor hypothermic portal perfusion techniques.
IVC Resection and Reconstruction
Risk factors for IVC resection include (i) complete obstruction of the caval lumen; (ii) densely adherent intracaval tumor; (iii) encasement of the great vessels by bulky disease; and (iv) direct caval wall invasion [16]. This has been the case in our patient.
In our patient we performed a two-step TVE. When complete IVC control is achieved, the first step is started, the infrarenal vena cava is resected by stapling. The left renal vein could be completely ligated and divided. Then, an extended longitudinal cavotomy allowed complete thrombus removal along the retrohepatic IVC (Fig. 11.4a, b). Then the IVC anterior wall was opened to a level of the right hepatic vein, and the IVC and right hepatic vein lumens were flushed with heparin and completely cleared of thrombus fragments. The retrohepatic IVC was resected to a level below the ostia of the right hepatic vein (Fig. 11.4c, d). Some centers did not perform IVC replacement, as chronic venous obstruction had created spontaneous retroperitoneal collaterals. In our case, we performed IVC replacement and reimplanted left renal vein into the prosthetic graft.
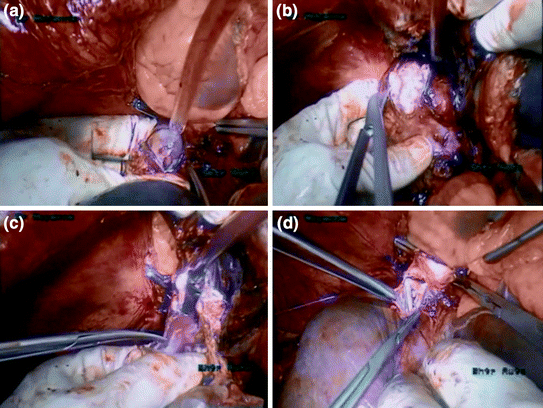

Fig. 11.4
Resection of the inferior vena cava. a Cavotomy at a level above the hepatic vein. b Cavotomy at a level below the hepatic vein. c Resection of the inferior vena cava. d Reconstruction of the inferior vena cava
To re-establish IVC reconstruction, we used a 20-mm diameter external ring-reinforced PTFE (polytetrafluoroethylene). PTFE is the preferred synthetic material when replacement is considered, as it has low thrombogenic potential and a high reported patency rate. Once the upper part of the graft was anastomosed to the proximal end of the cavotomy, the cranial clamp is then repositioned at a level below the hepatic veins (Fig. 11.5).
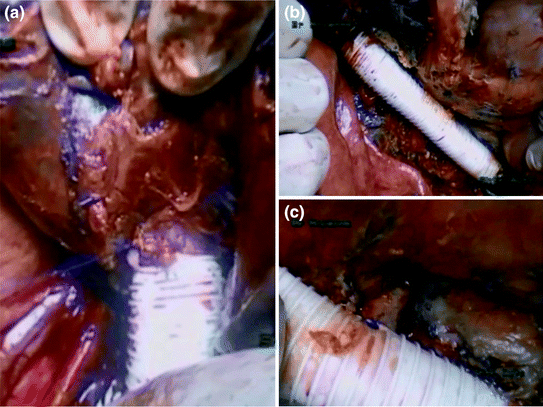

Fig. 11.5
Inferior vena cava reconstruction using a PTFE. a, b Inferior vena cava reconstruction. c Reimplantation of the left renal vein
Thereafter, the Pringle maneuver is released, and liver perfusion is restored. In a second step, radical en bloc resection of the right kidney and IVC was then performed. Then the resected caval segment is replaced with a synthetic graft in an end-to-end fashion.
In our case, the left renal vein stump was reconstructed by joining its free end to the interposition graft in an end-to-side fashion (Fig. 11.5c). Some other centers do not perform left renal vein reconstruction due to the presence of collateral veins development via the azygos-hemiazygos system that may preserve adequate drainage.
As for the right hepatic vein, two scenarios were possible: (i) the root of the right hepatic vein in the native vena cava remained untouched and this latter is patent, or (ii) the right hepatic vein was resected and its stump is reimplanted into the replaced vena cava. In our case, the root of the right hepatic vein in the native IVC was not resected and the right hepatic vein was completely patent after thrombus extraction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree