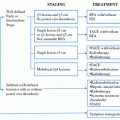
Fig. 1
Algorithm for the surveillance of hepatocellular carcinoma (HCC) (cited from the Group formed to establish the Guidelines for Evidence-Based Clinical Practice for the Treatment of Liver Cancer [89], with permission)
* High Risk Group includes patients with chronic hepatitis B or C virus or liver cirrhosis. *Super High Risk Group included patients with liver cirrhosis due to hepatitis B or C virus
1.1 Definition of Small HCC
There is no clear unified definition of “small” HCCs. In the staging system of the Liver Cancer Study Group of Japan, T1 tumor is defined as a single tumor measuring 2 cm or less in diameter without vascular invasion [3]. In the AJCC/UICC staging system, on the other hand, T1 is defined as a solitary tumor of any size without vascular invasion, although 5 cm is the cutoff between T2 and T3 for multinodular HCCs [4]. In general, local ablation therapy is indicated for three or fewer tumors measuring 3 cm or less in diameter, as suggested by several guidelines [2, 5, 6]. Considering all of the above, the general consensus for the definition of a small HCC would be a tumor measuring 3 cm or less in diameter.
1.2 Small HCC and Early HCC
Early HCC is a vaguely nodular and well-differentiated HCC, which is defined histologically. The size of an early HCC may be as small as 2 cm or less in diameter [3]. It grows by focally replacing the hepatocytes without destroying the lobular architecture. A more than twofold increase in the cell density is observed and portal tracts are present within the tumor [7–9]. Differential diagnosis of early HCC from a high-grade dysplastic nodule (DN), which is a precursor lesion of HCC [10], is problematic. Macroscopically, high-grade DN is a vaguely nodular, unencapsulated, and hypovascular tumor. Microscopically, it shows cytological and/or architectural atypia, but to a degree insufficient for the diagnosis of malignancy [11]. The most helpful morphological feature for the diagnosis of early HCC is the varying degree of tumor cell invasion of the portal tracts within the tumor, called stromal invasion [12–14]. Other features include loss of reticulin, sinusoidal capillarization, or neovascularization as evaluated by CD34 staining, Glypican 3 expression, and hepatocytic invasion of portal triads and septa as evidenced by CK7 expression [15–17]. Although it is usually difficult to differentiate early HCC from DN, recent studies suggest that contrast-enhanced ultrasonography (CEUS) may enable accurate differentiation between the two, as described later.
It should be noted that “small” HCC is not necessarily equivalent to “early” HCC. Early HCC is a distinct clinical entity with the term “early” justified by its association with a reduced risk of recurrence and death after hepatic resection as compared to the case for overt HCC [30]. Macroscopically, two types of small HCC have been described; one is the distinctly nodular type, which is well demarcated and frequently encapsulated and is not much different from overt HCC, while the other is only indistinctly or vaguely nodular. Tumor cell invasion of the portal vein and intrahepatic metastases in the vicinity of the tumor are observed in 27 and 10 %, respectively, in the distinct type but not in the indistinct type [6]. Therefore, only the latter types of small HCCs are defined as early HCCs. Although tumor size has been shown to be associated with vascular invasion, two previous studies reported a high incidence of microscopic vascular invasion even in small tumors: in 17 of 69 (25 %) HCCs measuring 2 cm or less in diameter [18], and 65 of 260 (25 %) HCCs measuring 3 cm or less in diameter [19].
2 Diagnosis
2.1 Contrast-Enhanced Ultrasonography
Microbubble contrast agent for sonography was first introduced by Matsuda et al. in 1986 [20]. It was made of carbon dioxide (CO2) and was initially injected into the hepatic artery as a contrast agent for sonographic angiography. Kudo et al. observed the vascular pattern changes from the very beginning of the CO2 injection until the time when all the CO2 was washed out from the entire liver, and classified them according to the type of hepatic nodules. They reported a higher sensitivity of detection of small HCCs as hypervascular nodules by this technique than by conventional angiography [21]. Later, SH U 508 (Levovist®), a contrast agent that is injected intravenously and can traverse the pulmonary capillary bed, was introduced [22]. However, the bubbles easily collapsed by ultrasound, making only transitory observation of the vascular pattern changes possible. More recently, a second-generation contrast agent, NC100100 (Sonazoid®) was developed by Nydomed Amersham [23]. This agent is stable, with Kupffer imaging in the post-vascular phase lasting for at least 3 h after the injection, and the agent has been shown to be well tolerated for multiple US scanning. Contrast-enhanced Ultrasonography (CEUS) using sonazoid has been shown to be useful for differentiation between HCC and DN. Overt HCCs show intratumoral vessels in the arterial phase, followed by tumor parenchymal staining in the portal phase. In the Kupffer phase, the tumor is visualized as a defect. In contrast, DN shows no blood signal in the arterial phase, followed by isoperfusion within the nodule in the portal phase, resulting in an iso- to hyperechoic pattern. In Kupffer phase, the nodule shows isoperfusion [24, 25].
Further, CEUS also helps in the preoperative prediction of tumor differentiation grades. Two recent studies evaluated the association between the enhancement patterns on CEUS and the histopathologic differentiation grades, and showed that well-differentiated HCCs showed significantly slower washout in the portal phase than moderately or poorly differentiated HCCs [26, 27].
2.2 Ethoxybenzyl-Magnetic Resonance Imaging
Gadolinium ethoxybenzyl diethylenetriamine penta-acetic acid (Gd-EOB-DTPA) is a newly developed contrast agent for magnetic reasonance imaging (MRI). It allows hemodynamic imaging as an extracellular agent, and also late hepatobiliary phase imaging by being accumulated in the hepatocytes 10–20 min after the injection [28]. A higher sensitivity of Ethoxybenzyl-Magnetic resonance imaging (EOB-MRI) to detect especially small lesions or tumors in the cirrhotic liver as compared to conventional computed tomography (CT) has been reported by several studies [29, 30].
Because of the low invasiveness of MRI as compared to angiography, most institutions have begun to adopt EOB-MRI as a preoperative diagnostic modality for HCC. Previously, popular methods such as angiography followed by lipiodol CT [31] and CT during arteriography/arterioportography [32] have been used less and less. The evidence-based guidelines also recommend either examination [2]. However, there is no established evidence to suggest which of these modalities might be the most accurate for the diagnosis of HCCs.
2.3 Intraoperative Ultrasonography
Intraoperative ultrasonography (IOUS) is an essential tool for liver surgery. It is useful not only for recognition of the vasculobiliary anatomy in relation to the tumor, as described below, but also for the detection of new lesions that were not recognized preoperatively. Zhang et al. reported the detection rates of HCC by various imaging modalities in 430 patients who underwent 553 hepatectomies between 1995 and 2002. The detection rate of the 430 and 110 HCCs in the patients who underwent primary and secondary hepatectomy, respectively, was the highest by IOUS (98 and 96 %, respectively). The detection rates by other preoperative imaging modalities, including ultrasonography, CT, angiography, lipiodol CT, and MRI ranged from 74 to 94 % and 73 to 93 % in those who underwent primary and secondary hepatectomy, respectively [33]. A more recent study suggested that intraoperative CEUS may allow even more accurate diagnosis of small HCCs than conventional IOUS, and also more complete surgical resection with a decreased rate of surgical margin-positive resection, due to the clear detection of the tumor margin afforded by the Kupffer-phase images [34].
3 Surgical Resection
Liver transplantation, which eliminates both HCCs and the background cirrhotic liver, may be the ultimate curative treatment for HCCs. Although the details are described in later chapters, the selection of transplantation for resectable small HCCs is controversial. Poon et al. reviewed the treatment outcomes in 136 patients with HCCs in a background of Child-Pugh A cirrhosis satisfying the Milan criteria (single tumor ≤5 cm in diameter, or two to three tumors, with none >3 cm in diameter) [35], and reported 5-year overall and recurrence-free survival rates of 70 and 36 %, respectively, after resection. Because a considerable proportion of the patients showed long-term recurrence-free survival and the majority of those with recurrence were eligible candidates for transplantation, they proposed hepatic resection as the first-line treatment and salvage liver transplantation after recurrence as a feasible strategy for patients with small HCCs and well-preserved liver function [36]. This proposal is also reasonable from the viewpoint of donor shortage.
HCCs tend to metastasize via the adjacent portal vein [37], which results in potential intrahepatic recurrence after hepatic resection. As mentioned above, small HCCs also show portal venous invasion, which is known to be associated with the prognosis [38]. Therefore, any resection should secure potential intrahepatic metastases. On the other hand, because major hepatic resection in a cirrhotic liver is associated with a high risk of postoperative liver failure, parenchyma-sparing resection is required. To overcome this pitfall, anatomic resection with removal of the entire liver parenchyma fed by the glissonian branches bearing the tumor was proposed. According to the expected residual liver function, various extents of resection, that is, hemihepatectomy, sectionectomy, or segmentectomy may be selected. To date, several studies have evaluated the outcomes of anatomic hepatic resection for HCCs. Another issue that needs to be considered in parenchyma-sparing liver resection is whether a tumor-free resection margin should be secured, especially when the tumor is attached to major vasculo-biliary structures. These two issues are discussed in the following sections.
3.1 Anatomic Versus Nonanatomic Resection
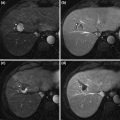
In anatomic resection, the liver parenchyma is dissected based on the portal venous anatomy. The concept of this type of hepatectomy is to remove the parenchyma fed by portal branches bearing the tumor, in order to eliminate potential intrahepatic metastases. Anatomic subsegmentectomy was first proposed by Makuuchi et al. in 1985 [39]. According to the size and location of the tumor, resection of complete Couinaud’s segment, part of a segment, or more than one segment extending to the adjacent region can be performed. The main feature of this procedure is dye injection into the portal venous branches bearing the tumor under the guidance of intrahepatic ultrasonography. From the liver surface, portal branches are punctured distal to the point of ligation. The stained surface of the liver is marked with electric cautery, followed by parenchymal dissection. It should be noted that hepatic veins appear on the dissected surface after complete resection in anatomic hepatic resection (Fig. 2). Anatomic subsegmentectomy is technically difficult, especially for segments 4, 7, and 8, as these locations are surrounded by major vascular structures and do not necessarily present a flat dissection plane [40]. To date, few studies have evaluated the short- and long-term outcomes of subsegmentectomy. [41, 42] Hasegawa et al. reported the long-term outcomes after anatomic and non-anatomic resection for solitary HCCs [43]. In this study, 210 patients were included and 84 of these (40 %) underwent subsegmentectomy. Both the overall and recurrence-free survival rates of the 156 patients who underwent anatomic resection, including subsegmentectomy, were significantly superior to those of the 54 patients who underwent non-anatomic limited resection or enucleation. Further, anatomic resection was one of the independent prognostic predictors of better overall and recurrence-free survivals.
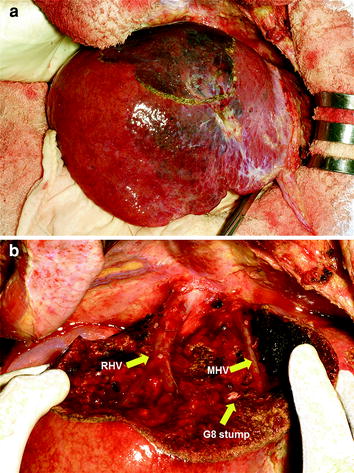

Fig. 2
Segment 8 visualized by staining with dye injected into the portal pedicle of segment 8 under ultrasonographic guidance (a), and dissected surface after resection (b). G8 stump, MHV, RHV: glissonian pedicle of segment 8, middle hepatic vein, and right hepatic vein, respectively
Survival benefit of anatomic resection in comparison to that of non-anatomic partial resection has also been reported in several other studies, although all types of anatomic resection in addition to subsegmentectomy are included and controversial results have also been reported (Table 1) [43–56]. In these studies, the superiority of anatomic resection was only demonstrated for selected patients with solitary HCC, small HCC, or HCC satisfying the Milan criteria. Several studies showed superiority of anatomic resection for selected patients based on liver function and/or the gross appearance type. Yamashita et al. reviewed the outcomes of 201 anatomic resections and 120 limited resections for solitary HCCs measuring less than 5 cm in diameter, and reported that anatomic resection was associated with better overall and recurrence-free survival rates in patients with liver damage A, but not with liver damage B [3, 48]. Yamamoto et al. and Ueno et al. reported better results after anatomic resection only for specific types of HCCs, such as nodular-type HCCs with extranodular growth, confluent multinodular-type HCCs, or invasive-type HCCs [45, 50].
Table 1
Summary of reports on the prognostic impact of anatomic resection
Authors | Year | N | Inclusion criteria | Results (in comparison to non-anatomic resection) |
|---|---|---|---|---|
Imamura et al. [44] | 1999 | 138 | <5 cm | Better RFS |
Yamamoto et al. [45] | 2001 | 204 | Solitary and <5 cm | Better OS only in nodular HCCs with extranodular growth |
Regimbeau et al. [46] | 2002 | 64 | Child Pugh A and ≦4 cm | Better OS and RFS |
Hasegawa et al. [43] | 2005 | 210 | Solitary | Better OS and RFS |
Kaibori et al. [47] | 2006 | 247 | HCV Ab (+) and HBs Ag (−) | Comparable OS and RFS |
Yamashita et al. [48] | 2007 | 321 | Solitary and <5 cm | Better OS and RFS in liver damage A Worse OS and RFS in liver damage B |
Wakai et al. [49] | 2007 | 158 | pT1 or pT2* | Better OS and RFS only in pT2 |
Ueno et al. [50] | 2008 | 116 | ≦3 cm and up to 3 nodules | Better RFS only in non-boundary gross type** |
Tanaka et al. [51] | 2008 | 125 | Solitary | Comparable OS and RFS |
Eguchi et al. [52] | 2008 | 5781 | Solitary | Better RFS only in HC of 2 to 5 cm diameter |
Kang et al. [53] | 2010 | 167 | Child-Pugh A and Solitary and ≦4 cm | Comparable OS and RFS |
Kamiyama et al. [54] | 2010 | 322 | Satisfying Milan’s criteria [35] | Better OS and RFS |
Dahiya et al. [55] | 2010 | 373 | Solitary and ≦5 cm | Comparable OS and RFS |
3.2 Hepatic Resection Margin
It is controversial whether HCCs should be resected with a free resection margin. A wide resection margin has been recommended by several studies, including one prospective randomized study, that showed that lower recurrence rates or better prognoses were associated with a wide resection margin as compared to a narrow or zero resection margin [56–62]. Other studies showed no correlation between the width of the resection-free margin and the prognosis [63–70]. The most optimal width of the resection margin is still not clear. To discuss this issue, the morphology of HCCs, especially the presence of a tumor capsule, which is characteristic of well-demarcated HCCs, and other background factors would also need to be considered. Historically, Kanematsu et al. investigated the outcomes of limited hepatic resection with less than 1 cm resection margin for HCCs in 37 patients with cirrhotic livers with severely impaired liver function with a mean ICG‐R15 of 31%, and reported a 5-year survival rate of 33 % [71]. They evaluated the risk factors for postoperative recurrence and suggested that the presence of a fibrous capsule was associated with a lower risk of recurrence, and that limited resection is a feasible option for cirrhotic liver. Matsutani et al. reported that a wide margin of 1.0 cm or more was associated with a longer survival as compared with a narrow margin of less than 1.0 cm, only in the case of small HCCs measuring 2.0 cm or less in diameter [57]. On the other hand, Shimada et al. proposed that a surgical margin of 1.0 cm or more must be secured in young patients without hepatitis C virus infection and/or patients with a tumor diameter of 2.5 cm or more [62]. HCCs are typically covered with a capsule and nucleation of the tumor is often possible. Especially when the tumor is adjacent to major vascular structures in a cirrhotic liver, hepatic resection exposing the tumor is inevitable to preserve the vascular structures. It is also important to evaluate the recurrence pattern, especially whether the intrahepatic recurrence is along the transection surface or not, for evaluation of the usefulness of wide-margin hepatectomy. The transection margin recurrence rate of 29.5 % (13/44) after resection with a narrow margin reported by Shi was rather high [61], while Torzilli reported that they had no experience of a single case of cut-edge recurrence over a median follow up period of 24 months after tumor-exposing hepatic resection [72]. Poon et al. also reported that recurrence occurred at a distal segment or multiple segments rather than at the resection line in most cases, even after margin-positive resection, in their study of 150 and 138 patients who underwent resections with a narrow (<1 cm) and wide (≧1 cm) resection margin, respectively [68].
3.3 Intrahepatic Metastases and Multicentric Occurrence
As mentioned above, anatomic subsegmentectomy is aimed at eliminating potential Intrahepatic Metastases (IM). Another type of intrahepatic recurrence, that is Multicentric Occurrence (MO), however, cannot be prevented by this type of resection. IM is defined by the Liver Cancer Study Group of Japan as: (1) tumors clearly growing from portal venous thrombi, (2) tumors surrounding a large main tumor with multiple satellite nodules, or (3) a small solitary tumor near the main tumor that is histologically similar or less differentiated than the main tumor [3]. A previous study showed that a short recurrence-free interval, usually less than 1–2 years, may suggest IM rather than MO, [63, 73, 74] and that MO is associated with HCV rather than HBV infection [75]. This may explain the no-superiority of anatomic resection in HCV-positive patients noted in Kaibori’s study [47]. However, differentiation of IM from MO is difficult by the usual radiologic and histopathologic evaluations alone [76, 77]. Although genetic analyses to evaluate tumor clonality have been tried, their usefulness was shown only in selected cases, such as HBV-positive patients, female patients, or non-cirrhotic liver patients [78–81].
3.4 Laparoscopic Hepatic Resection
Although the details are referred to in a later chapter, laparoscopic hepatic resection for HCC is one of the treatment options for patients with severe liver cirrhosis. In general, experience with laparoscopic hepatectomy is still too limited to serve as evidence of their suitability, especially for malignant tumors. The Louisville statement made in the consensus conference by 45 experts held in October 2008 proposed that the most suitable candidates among HCC patients for laparoscopic hepatectomy are those with solitary lesions measuring 5 cm or less in diameter, located in peripheral segments [82]. Several surgeons advocate laparoscopic resection for HCCs, especially for those occurring in a background of liver cirrhosis, because of its low invasiveness, lesser need for liver mobilization, and lower volume needed of intravenous fluid replacement due to the minimized insensible fluid loss during the operation as compared with that during open liver resection. Fluid accumulation in the third space would decrease, which would result in a reduced risk of prolonged postoperative ascites [83–86]. Keneko et al. compared the degree of invasiveness between laparoscopic and open hepatectomy for HCCs based on the Estimation of Physiologic Ability and Surgical Stress [87, 88] and showed significantly less surgical stress and smaller comprehensive risk scores in the laparoscopic hepatectomy group. They proposed that laparoscopic hepatectomy for HCCs represents an intermediate option between local ablation therapy and conventional hepatectomy, because it is superior to ablation from the point of view of its allowing complete resection of the tumor, but inferior to open hepatectomy in terms of anatomic resection, while being less invasive [86].
4 Conclusion
Regular checkups of high-risk populations for HCC would be expected to increase the likelihood of detection of small HCCs, and consequently increase the percentage of suitable candidates for curative treatment of HCC. It should be noted that small HCCs are not necessarily equivalent to early HCCs, since approximately 20 % of small HCCs show evidence of microscopic vascular invasion. Anatomic hepatic resection or resection with a wide margin, to the maximum extent permitted by the expected residual liver function, would be the treatment of first choice. On the other hand, liver transplantation would be a reasonable option of first choice for patients with severe cirrhosis. Laparoscopic hepatectomy is a useful procedure due to its low invasiveness, however, further experience is required before it is accorded a place as standard treatment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree