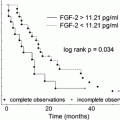
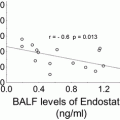
Patients rs1800734
Patients rs2303425
Controls rs1800734
Control rs2303425
Total sample (n)
422
422
511
486
Gender
Male n (%)
322 (76)
324 (77)
287 (56)
270 (56)
Female n (%)
100 (24)
98 (23)
224 (44)
216 (44)
Age (year; range)
Total group
64 ± 10 (24–88)
67 ± 7 (22–85)
Male
64 ± 9 (24–85)
59 ± 8 (22–85)
Female
63 ± 12 (25–88)
61 ± 10 (23–81)
Histology n (%)
292 (100 %)
Adenocarcinoma
121 (41 %)
Small cell carcinoma
50 (17 %)
Non-small cell carcinoma
22 (8 %)
Spinocellular carcinoma
65 (22 %)
Epidermoid carcinoma
34 (12 %)
2.2 Genotyping Analysis
Genomic DNA was isolated using a standard phenol-chloroform extraction. Two SNPs located in the promoter regions of MMR genes were chosen for the study. The SNPs were determined by a PCR-RFLP method. The following primer sequences were used for genotyping of rs1800734 located in hMLH1 gene: forward – 5′- TGA CTG GCA TTC AAG CTG TC-3′ and reverse – 5′-TTC ACC ACT GTC TCG TCC AG-3′. The PCR reaction was done in a total volume of 25 μl, containing 1 μl of genomic DNA, 1 μl of each primer, 9.5 μl of redistilled water, and 12.5 μl of DreamTaq Green PCR Master Mix (BIOGEN Praha s.r.o., Czech Republic). The PCR cycle conditions consisted of an initial denaturation step at 94 °C for 5 min, followed by 35 cycles of 40 s each at 94 °C, 40 s at 56 °C, 1 min at 72 °C, and a final extension step at 72 °C for 7 min. The PCR product was digested with an appropriate volume of PvuII restriction endonuclease. Primer sequences used for genotyping rs2303425 located in hMSH2 gene were: forward- 5′-AGG CAT GCG CAG TAG CTA AA-3′ and reverse-5′-CCC ACA CCC ACT AAG CTG TT-3′. PCR reaction was performed in a total volume of 13 μl, containing 1 μl DNA, and half of the other ingredients of Master Mix used for polymorphism rs1800734. The PCR cycle conditions were the same as those for the polymorphism rs1800734 above outlined. Restriction endonuclease used for PCR product digestion was BseNI. Digested PCR products were analyzed by gel electrophoresis (3 % agarose) and ethidium bromide staining for visualisation under UV light. Following fragment products were seen after visualisation: rs1800734 hMLH1 gene, wild type genotype WT = 69 bp + 145 bp, heterozygous genotype Het = 69 bp + 145 bp + 214 bp, variant genotype Var = 214 bp, and for rs2303425 hMSH2 gene WT = 190 bp, Het = 190 bp + 118 bp + 72 bp, Var = 118 bp + 72 bp.
2.3 Statistical Analysis
The Fisher exact test was used to determine the significance of differences from the Hardy-Weinberg equilibrium and the allelic association between cases and controls. Odds ration (OR) and 95 % confidence intervals (95 % CI) were calculated to estimate the strength of associations between different genotypes in patients and controls. A p-value <0.05 was considered statistically significant. The Fisher exact test was also used to examine the association between genotypes and cancer risk in two genetic models: dominant and recessive. Statistical analysis of the association between a mutual genotype combination of rs1800734 and rs2303425 polymorphisms and risk of lung cancer development was carried out by the Chi square test (χ2). Statistical calculations were performed using Microsoft Excel and SNP & Variation Suite ver. 7.x6.11 software.
3 Results
Minor allele frequencies (MAF) and their association with lung cancer risk are presented in Table 2. We found only one statistically significant association between minor allele A and cancer risk in the group of women (OR = 0.66; 95 % CI = 0.45–0.99; p = 0.043). The frequency of variant alleles of polymorphisms in the present study were compared with previous published reports. Table 3 describes the main results concerning the associations of genotype rs1800734 and rs2303425 polymorphisms with the risk of lung cancer evaluated in two genetic models: dominant and recessive. The evaluation of cancer risk in relation to genotypes has shown two distinct associations of rs1800734 polymorphism in the dominant genetic model. One was of polymorphism rs18000734 among lung cancer patients, where the presence of at least one variant allele A in genotype (genotype GA and AA) significantly decreased the risk of lung cancer by 1.4 times (OR = 1.40; 95 % CI = 1.08–1.82; p = 0.01). Assessing the risk of lung cancer in regard to gender showed an equally significant result for the rs1800734 polymorphism in women (OR = 2.00; 95 % CI = 1.23–3.25; p = 0.006). No significant association between rs1800734 polymorphism and risk of lung cancer was found in the recessive genetic model.
Table 2
Minor allele frequencies and their association with lung cancer risk
Group | Gene | Minor allele | MAF in patients | MAF in controls | Fischer’s exact p allelic associations | Minor allele OR (95 % CI) |
|---|---|---|---|---|---|---|
hMLH1 | A | |||||
All | 0.237 | 0.268 | 0.14 | 0.85 (0.69–1.05) | ||
Male | 0.245 | 0.254 | 0.74 | 0.95 (0.74–1.24) | ||
Female | 0.210 | 0.286 | 0.04* | 0.67 (0.45–0.99) | ||
hMSH2 | C | |||||
All | 0.192 | 0.189 | 0.91 | 1.02 (0.80–1.29) | ||
Male | 0.187 | 0.198 | 0.66 | 0.93 (0.69–1.24) | ||
Female | 0.209 | 0.178 | 0.38 | 1.22 (0.80–1.86) | ||
Table 3
Associations between genotypes – rs1800734 and rs2303425 polymorphisms with the risk of lung cancer development in two genetic models
Dominant model (AA + GA vs. GG) | Recessive model (GG + GA vs. AA) | ||||||
hMLH1 genotypes | Group | Patients n | Controls n | OR (95 % CI) | p | OR (95 % CI) | p |
All | |||||||
G_G | 250 | 260 | 1.40 (1.08–1.82) | ||||
G_A | 144 | 228 | 0.71 (0.55–0.93) | 0.01* | |||
A_A | 28 | 23 | 1.51 (0.86–2.66) | 0.19 | |||
Male
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |||||||