Increase in serum creatinine level (mg/dL)
Multivariable OR (95 % CI)
Area under ROC curve
Increase in total cost
0.3
4.1 (3.1–5.5)
0.84
$4,886
0.5
6.5 (5.0–8.5)
0.86
$7,499
1.0
9.7 (7.1–13.2)
0.84
$13,200
2.0
16.4 (10.3–26)
0.83
$22,023
Definitions
Historically, the definition of AKI has included 35 different terms in the literature [7, 8]. This lack of standardization has greatly impeded the understanding of the epidemiology of kidney dysfunction. Most recently, nephrologists have proposed using the term “acute kidney injury” as a simple and direct description of the process, one that patients can readily understand and one that includes mild and moderate kidney dysfunction along with outright kidney failure. Additionally, the term injury is thought to better represent the pathologic changes occurring in the kidney parenchyma [7]. In 2004, the Acute Dialysis Quality Initiative (ADQI) workgroup published the RIFLE criteria as a method of staging acute kidney injury. They created an acronym for 3 levels of renal dysfunction (Risk of renal dysfunction, Injury to the kidney, Failure of kidney function) and 2 levels of outcome (Loss of kidney function and End-stage kidney disease). The dysfunction criteria are based on a relative rise in creatinine, the absolute level of urine output, or both. The failure category uses serum creatinine ≥4 mg/dL, to account for the severity of acute renal disease in patients with chronic renal disease whose relative increase in creatinine may not otherwise reflect AKI [9] (Table 32.2).
Table 32.2
Staging for AKI-RIFLE and AKIN criteria
RIFLE | SCr criteria | UOP criteria | AKIN stage | SCr criteria | UOP criteria | |
|---|---|---|---|---|---|---|
 | R | ↑ SCr × 1.5 | <0.5 mL/kg/h × 6 h | 1 | ↑ in SCr ≥0.3 mg/dL or ↑ ≥150–200 % from baseline (1.5- to 2-fold) | <0.5 mL/kg/h for >8 h |
I | ↑ SCr × 2 | <0.5 mL/kg/h × 12 h | 2 | ↑ in SCr to >200–300 % from baseline (>2- to 3-fold) | <0.5 mL/kg/h for >12 h | |
F | ↑ SCr × 3, or SCr ≥4 mg/dL with an acute rise of at least 0.5 mg/dL | <0.5 mL/kg/h × 24 h or anuria × 12 h | 3 | ↑ in SCr to >300 % (3-fold) from baseline or SCr ≥4 mg/dL with an acute rise of at least 0.5 mg/dL | <0.5 mL/kg/h × 24 h or anuria × 12 h | |
L | Persistent loss of kidney function for >4 weeks | |||||
E | Persistent loss of kidney function for >3 months |
The predictive value of RIFLE criteria for mortality is a near-linear relationship between the renal dysfunction by RIFLE criteria and hospital mortality. In a retrospective, single-center trial of 20,126 patients, Uchino et al. found that 9.1 % of all patients fell into the risk category, 5.2 % into the injury category, and 3.7 % into the failure category with hospital mortality of 4.4 % for normal patients, 15.1 % for patients falling in the “risk” group, 29.2 % for patients falling in the “injury” group, and 41.1 % for patients falling in the “failure” group. Multivariate logistic regression analysis indicated that RIFLE criteria were predictive for hospital mortality [11]. The RIFLE criteria have been validated in the elderly and in the critically ill [12, 13].
Risk Factors
The etiology of AKI is often multifactorial in geriatric patients. Age-related changes in renal function make the elderly more susceptible to AKI at baseline [14, 15]. In the trauma setting, hypovolemia from under-resuscitation and shock from hemorrhage play important roles in increasing the risk of AKI. Comorbidities such as diabetes, hypertension, cardiovascular disease, congestive heart failure, and especially preexisting chronic kidney disease are additional independent risk factors for developing, and not recovering from, an episode of AKI. Furthermore, the treatment of these conditions often requires the use of nephrotoxic drugs which further enhance the risk of AKI [12]. Intravascular volume depletion from preexisting congestive heart failure or liver disease increases susceptibility to AKI in the setting of trauma and hospitalization [15]. Contrast-induced nephropathy is thought to be a major cause of kidney injury in elderly patients, although the incidence is unknown. Data from the cardiac literature suggests that age greater than 75 years is an independent risk factor for AKI after percutaneous coronary intervention [16]. The risk is further increased in the setting of chronic kidney disease [10, 15]. Medication-induced nephrotoxicity is another major cause of AKI in the elderly who tend to be on multiple medications and have decreased renal clearance due to age-related changes [15, 17]. Rhabdomyolysis which can be seen in severe trauma may put elderly patients at risk for AKI from deposition of myoglobin, hemoglobin, or light chains [15]. Geriatric trauma patients are also at risk for infection from numerous sources, which is another key risk factor for AKI in this population. Lastly, obstructive nephropathy can also contribute to AKI. Older male patients are at particular risk for obstruction from prostatic disease [15] (Fig. 32.1).
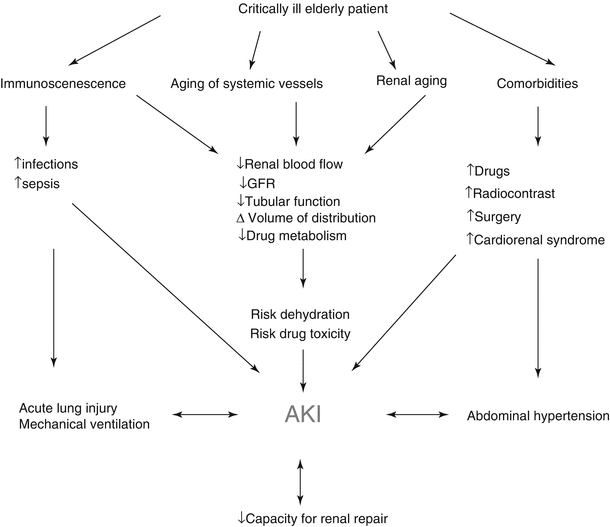

Fig. 32.1
Factors contributing to AKI (Adapted from [12]. Reproduced, with permission, from Springer Science + Business Media)
Age-Related Changes of the Kidney and Risk of AKI in the Elderly
Many age-dependent changes occur in the kidney. The glomerular filtration rate (GFR) falls by 10 % per decade after age 40 [14, 18, 19]. This is due to age-related functional reductions in the glomerular capillary plasma flow rate, reduction in afferent arteriolar resistance, and increase in glomerular capillary hydraulic pressure. However, serum creatinine levels in elderly patients can remain within the normal range despite gross reductions in GFR [14, 20]. Serum creatinine is reflective of muscle mass and consumption of protein load both of which are decreased in elderly patients thus making it an unreliable measure of renal functional reserve in this patient population. Structural changes within the kidney also occur with aging and result in the loss of renal mass, hyalinization of afferent arterioles, glomerular arterioles, and an increase in the percentage of sclerotic glomeruli and tubulointerstitial fibrosis. By age 70, the loss in renal mass measured by functioning cortical glomeruli due to ischemia ranges between 30 and 50 % [15, 19, 21]. Changes in the activity of the renin-angiotensin and nitric oxide systems further lead to loss of urinary concentration and diluting ability, inability to conserve sodium, and decreased plasma renin and aldosterone levels. These changes lead to volume depletion and dehydration, thus making elderly patients more susceptible to AKI when exposed to other risk factors such as trauma, sepsis, or nephrotoxins [19, 21]. With aging, there is also altered activity and responsiveness to vasoactive stimuli. Responses to vasoconstrictor stimuli are enhanced, while vasodilatory responses are impaired which further increases sensitivity to risk factors for AKI. Renal function becomes more dependent on prostaglandin-mediated afferent arteriolar vasodilation with advancing age [19–21]. In the elderly, the traditional features of prerenal AKI such as low urine sodium, low fractional excretion of sodium, low fractional excretion of urea, high urine osmolality, and an elevated blood urea nitrogen/serum creatinine ration are less reliable due to age-related changes in fluid and electrolyte homeostasis. Urine sodium may be over 20 meq/l and urine osmolality less than 500 mOsmol/kg despite intravascular hypovolemia. The inability to concentrate urine may cause inappropriately high urine output. With aging, total body water as a fraction of body weight is also decreased further adding to the risk of AKI [15] (Fig. 32.2).
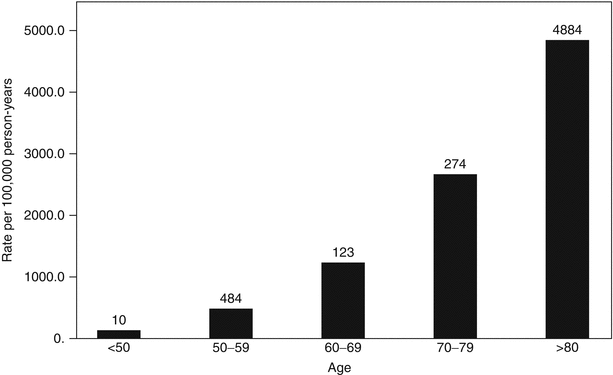

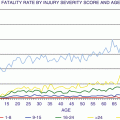
Fig. 32.2
Age and incidence of community-acquired AKI [1] (Reprinted, with permission, from Nature Publishing)
Chronic Kidney Disease (CKD) and Risk of AKI in the Elderly
Elderly patients with CKD are most susceptible to develop AKI with poor renal recovery because of their decreased renal reserve. Age older than 65 is a risk factor for nonrecovery from AKI and even progression to advanced-stage CKD [17, 22]. Elderly patients with estimated glomerular filtration rate (eGFR) of 45–59 mL/min/1.73 m2 are at higher risk for AKI compared with their counterparts with eGFR >60 mL/min/1.73 m2 [1]. Recent data from the Atherosclerosis Risk in Communities Study demonstrated that the adjusted risk of AKI approximately doubles as eGFR declines from 60 to 45 mL/min/1.73 m2 [9, 23].
Among patients with AKI and baseline GFR <45 mL/min/1.73 m2, almost half develop ESRD within 30 days, and even those who do not are more likely to develop ESRD within the subsequent 4 years. The annualized risk of ESRD increases to 7–9 % if the AKI occurs in an individual with a preexisting history of CKD [24–26] (Fig. 32.3).
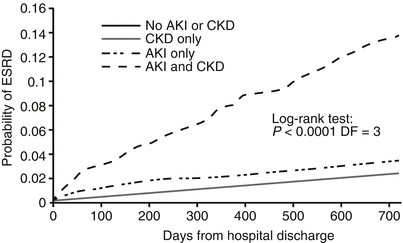

Fig. 32.3
Relationship of AKI and CKD [25] (Reprinted, with permission, from the American Society of Nephrology)
Hypovolemia and Risk of AKI in the Elderly
Intravascular hypovolemia results in AKI due to renal hypoperfusion from decreased effective arterial blood volume. Baseline intravascular hypovolemia from congestive heart disease or liver disease increases the risk of ischemic and nephrotoxic insults in elderly patients who already have physiologic age-related changes as outlined above [14]. Traumatic hemorrhage, third spacing of fluids after operative interventions, and sepsis may result in intravascular volume depletion which increases the risk of AKI [15, 27].
Infection
Elderly are at increased risk for healthcare-associated infections [28, 29], and they also have an increased risk of developing AKI from sepsis [12]. The BEST Kidney investigators reported in their prospective observational study of over 29,000 ICU patients that nearly half of the 5.7 % who developed AKI had septic shock as a likely etiology [4, 30]. Septic AKI is emerging as a separate entity with distinct pathophysiology and outcomes [27].
Drugs and Dye and the Risk of AKI in the Elderly
Medication use may play a significant role in AKI in the elderly. Diuretics and angiotensin-converting enzyme inhibitors used to treat hypertension and congestive heart failure deplete volume which can cause acute kidney injury. NSAIDs are associated with acute tubular necrosis or acute interstitial nephritis. NSAIDs have a longer half-life in the elderly and alter renal hemodynamics by inhibiting the production of vasodilatory prostaglandins and thus increase risk of acute kidney injury [17]. In a nursing home population, NSAIDs were found to increase the risk of AKI by 13 % [31]. Elderly patients also tend to have decreased body mass which increases the risk of AKI associated with NSAID use. Hypersensitivity to medications causes acute interstitial nephritis, a less common cause of AKI. Antibiotics especially penicillins, cephalosporins, and sulfonamides are often implicated.
Contrast-induced nephropathy is a major cause of AKI in hospitalized elderly patients [10, 32, 33]. Older patients with history of CKD particularly have an increased risk of developing CIN. However, recent studies suggest that this may not be the case in trauma patients. McGillicuddy et al. conducted a retrospective review of 1,371 trauma patients and found that intravenous contrast for CT did not result in higher rates of AKI [34]. In another retrospective review of 571 trauma patients admitted to the ICU at a level I trauma center for 48 h, Kim et al. found that nearly 30 % developed AKI. Age over 65years and Injury Severity Score greater than 25 were independent predictors of AKI on multivariate analysis. However, IV contrast did not predict AKI in these patients nor in subgroup analysis of patients >65 years, those who were hypotensive on admission or those who were severely injured (ISS >25) [35].
Obstruction
Postrenal obstruction occurs between the renal pelvis and the urethra. AKI from postrenal obstruction typically occurs in men from benign prostatic hypertrophy or prostate cancer. In women, retroperitoneal and pelvic malignancies are the most important cause of AKI due to obstruction. Space-occupying retroperitoneal lesions such as hematomas or abscesses can also cause ureteral or bladder obstruction in trauma patients. Imaging is essential for diagnosis – ultrasound, CT, or rarely intravenous pyelogram [15].
Workup
A detailed history and physical exam focusing on comorbidities, especially diabetes, hypertension and vasculitides, baseline renal function, and current medications, are essential to assessing the risk for AKI in any given patient. Physical exam should focus on the assessment of volume status and signs of uremia. Laboratory studies should include standard serum complete blood counts, chemistry panels, urine electrolytes, and examination for sediment. Typically a renal ultrasound is used to evaluate for obstructive causes. Rarely, a renal biopsy is necessary to truly establish the diagnosis especially when the cause of AKI is multifactorial [15].
Treatment
Aggressive fluid resuscitation may be needed to correct prerenal causes of AKI. This should be balanced with other conditions such as pulmonary edema, congestive heart failure, and elevated abdominal compartment pressures, which may limit how much volume an individual patient can tolerate. A critical step in treating AKI is to avoid exposure to nephrotoxins and minimize repeated episodes of AKI, which increase the risk of developing oliguric chronic renal failure especially in the elderly.
Diuretics in ARF
The physiologic argument to use diuretics in critically ill patients with AKI is that diuretics increase flow of urine and renal perfusion which reduces continual glomerular injury. Loop diuretics are also thought to decrease metabolic demand of renal tubular cells and reduce oxygen consumption [36]. Furthermore, some papers have shown that oliguric AKI is associated with higher mortality than nonoliguric AKI [36]. However, Karajala et al. conducted a systematic review in 2009 evaluating the role of diuretics in treating AKI and found that diuretics, while useful in managing volume status, do not improve mortality, decrease the need for renal replacement therapy (RRT), or reduce the time for recovery from AKI. Diuretics have a role, in the setting of oliguric AKI, if systemic complications of volume overload such as pulmonary edema are manifested [10, 37].
Indication for RRT
There are several indications for RRT in critically ill patients. RRT can correct electrolyte imbalances, acid/base disturbances, drug toxicity, fluid overload, and uremia [38]. Examples of conditions that may improve with renal support include congestive heart failure, respiratory acidosis from adult respiratory distress syndrome, liver failure, pancreatitis, fluid management in multi-organ failure, lactic acidosis, crush injury, tumor lysis syndrome, and possibly cytokine removal in sepsis [39, 40].
Initiation of RRT in an oliguric patient generally requires evidence of severe hyperkalemia (K >6.5 or rapidly rising levels), severe pulmonary edema, severe acidemia (pH <7.1), uremia, hyponatremia, hypernatremia, hyperthermia, and overdose although specific guidelines for thresholds are lacking [41]. Continuous renal replacement therapy (CRRT), rather than intermittent hemodialysis (IHD), is preferred for critically ill patients with hemodynamic instability. Non-renal indications for CRRT are being investigated. Several studies suggest that high-volume hemofiltration may affect levels of inflammatory mediators in sepsis and ARDS although the mechanisms are as yet unclear [39, 40, 42].
Principles of Renal Replacement Therapy
RRT modalities are categorized by mechanisms of fluid and solute removal and by the intermittent versus continuous nature of treatment. Given the lack of definitive outcome data for RRT modality in AKI, current practice is largely dictated by local resources and physician experience.
Fluid Removal – Ultrafiltration
Fluid removal is accomplished through ultrafiltration (UF) in all RRT methods with the exception of peritoneal dialysis (PD). UF uses a pressure gradient to drive fluid across a semipermeable membrane. Factors affecting the UF rate are the transmembrane pressure gradient, membrane water permeability, and membrane surface area.
Solute Removal
The two primary mechanisms of solute removal are diffusion and convection. In hemodialysis, solutes are cleared by diffusion. Diffusion is the movement of a solute from a higher to a lower concentration across a semipermeable membrane. Diffusion is most effective with low molecular weight molecules (<500 Da). The dialysate fluid, which generally contains sodium, bicarbonate, chloride, magnesium, and calcium, runs countercurrent to blood flow, thus maximizing the concentration gradient. The factors affecting the rate of solute clearance are solute molecular weight, flow rates of blood and dialysate, dialysis duration, concentration gradient across the membrane, membrane surface area, and membrane permeability.
Convection, the primary mechanism of solute clearance in hemofiltration, occurs when solutes are “dragged” with water during ultrafiltration. Solutes eliminated by convection include both low molecular weight molecules, such as potassium, phosphates, creatinine, and blood urea nitrogen (BUN), as well as medium molecular weight molecules up to 40,000 Da. Solute clearance is primarily dependent on the ultrafiltration rate, the ultrafiltration coefficient of the membrane, and the sieving coefficient of the solute that is inversely proportional to the molecular weight.
RRT Modalities
Intermittent renal replacement modalities are IHD and sustained low-efficiency dialysis (SLED), also referred to as extended daily dialysis (EDD). The continuous modalities are peritoneal dialysis (PD) and CRRT, which exists in various forms using ultrafiltration, hemodialysis, hemofiltration, hemodiafiltration, and combinations of these. The three primary modalities of RRT used to treat AKI are IHD, CRRT, and SLED. PD, a common modality in chronic kidney disease, is generally not used in the acute setting as it carries an increased risk of peritonitis, cannot be used in patients with recent abdominal surgery or abdominal sepsis, gives insufficient solute clearance in catabolic patients, and reduces respiratory function through impedance of diaphragmatic excursion.
Intermittent Hemodialysis
IHD uses diffusion for solute removal and ultrafiltration for volume removal. In AKI, it is generally performed 3–4 times/week, around 4 h per session, with blood flow rates of 200–300 mL/min and dialysate flow rates of 500–800 mL/min [43]. The advantages include swift solute and volume removal, relatively low cost and complexity, and relatively small anticoagulation requirements due to rapid flow rates. Its primary disadvantage is the risk of hypotension which may be seen in as many as 10 % of patients receiving IHD [43]. The rapid movement of solutes from the extravascular space can also cause cerebral edema, thus IHD is contraindicated in patients with head trauma or hepatic encephalopathy.
Continuous Renal Replacement Therapies
CRRT includes a variety of modalities that use ultrafiltration and may use convection, diffusion, or both. Treatment is 24 h/day with a blood flow of 100–200 mL/min and a dialysate flow of 17–34 mL/min in the case of diffusive technologies [43]. The advantage is a slow shift in both fluid and solutes allowing for better hemodynamic stability and more precise solute concentration control. The gradual nature of solute removal in CRRT makes it less likely to cause cerebral edema [44]. CRRT also has greater cumulative solute removal than IHD due to the longer treatment time.
Replacement solution supplants the ultrafiltrate continuously removed by hemofiltration and hemodiafiltration. Buffers used in the replacement solution are lactate, bicarbonate, or citrate. Lactate and citrate are metabolized by the liver and muscles to produce bicarbonate, which is easily tolerated, but can be unstable in solution. Commercially available bicarbonate solutions are manufactured with a 2-compartment bag to prevent carbonate precipitation during storage. Lactate is stable in replacement solution; however, it may contribute to an existing lactic acidosis in patients with sepsis or liver failure [45]. Citrate provides regional anticoagulation of the hemofilter. The choice of parameters within CVVH offers some flexibility for patients with differing underlying processes [39]. Citrate is successfully used in patients at risk of bleeding, while bicarbonate-based replacement solution is preferred in those with lactic acidosis or liver failure [46].
Slow Low-Efficiency Dialysis/Extended Daily Dialysis
SLED can use the same hemodialysis machines as IHD, but runs for longer periods at slower rates. A usual treatment runs for 6–12 h, with blood flow rate of 200 mL/min, and dialysate flow rate of 300 mL/min. It combines many of the advantages of IHD and CRRT. It is relatively low cost and low complexity since it uses the same technology as IHD; however, it also has the advantages of gradual fluid and solute removal and high total solute removal. In addition, because it is not continuous, other diagnostic and therapeutic procedures can occur between treatments.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree