Stage
N
Treatment
6-Year EFS%
6-Year survival
1
41
S + BEP
95
95.1
2
16
S + BEP
87.5
93.8
3
58
S + HDP/EB vs. BEP
96.6
97.3
4
16
S + HDP/EB vs. BEP
86.7
93.3
Tumor volume is one of the most important determining factors for prognosis [34, 37]. Malignant germ cell neoplasms are highly sensitive to platinum-based chemotherapy and can spread via the lymphatics, bloodstream, or by intraperitoneal dissemination; however, nodal involvement is less common, as these neoplasms are known to spread hematogenously to the liver and lungs. Females with metastatic or incompletely resected disease favor a poor prognosis. Even without randomized trials, there has been indirect evidence that patients with malignant ovarian germ cell tumors who have optimally debulked disease (defined as all areas of residual disease less than 1 cm) have a higher remission rate from chemotherapy and have better long-term outcomes than those with bulky unresectable disease [29, 32, 34, 37]. The majority of adolescents with ovarian germ cell tumors should undergo maximal surgical cytoreduction before starting chemotherapy. With modern cisplatin-based adjuvant chemotherapy, approximately 80 % of patients who present with advanced disease will be long-term survivors, even if they have residual disease remaining after cytoreductive surgery [34, 37, 42, 43]. This treatment regimen is tolerated well by the pediatric population, however, well-known side effects can still be observed. Etoposide can lead to a secondary malignancy such as an acute leukemia, while bleomycin has a 1 % risk of pulmonary fibrosis.
9.5.7 Menstruation/ Fertility
After chemotherapy, it is reported that at least 80 % of these patients will resume normal menstrual function [39, 42, 43]. In those who become pregnant, there is no apparent increase in pregnancy complications. Utilization of oocyte donors has enabled patients with a uterus and no ovaries to become pregnant and carry a child. Cryopreservation of fertilized eggs, ovarian tissue, and oocytes has allowed patients of a young age to plan for future fertility (see Sect. 9.10 below).
9.6 Ovarian Sex Cord Stromal Tumors
Ovarian malignancy comprises 1 % of childhood cancer, with sex cord stromal tumors comprising 7 % overall, and approximately 5–12 % of pediatric ovarian neoplasms [44]. The most common presenting types are juvenile granulosa cell tumors which present in 7–8 % of girls under 20 years old, followed by Sertoli-Leydig cell tumors presenting in 1–2 %. Classic clinical presentations include abdominal pain or distension, an abdominal mass or gastrointestinal symptoms. Sex cord stromal tumors are unique, in that, many patients may present with clinical evidence of sex hormone production with isosexual precocious puberty such as breast enlargement, galactorrhea, vaginal bleeding, virilization, or primary/secondary amenorrhea.
9.6.1 Diagnosis
Any patient with suspected ovarian malignancy should be screened for serum tumor markers. These include alpha fetoprotein (AFP), beta-human chorionic gonadotropin (β-hCG), carcinoembryonic antigen (CEA), cancer antigen 125 (CA-125), inhibin A and B, lactate dehydrogenase (LDH) and serum calcium. Measurement of inhibin B may be more specific, however, it may not be elevated in pre-pubertal children. These tumors may also secrete mullerian inhibitory substance (MIS). It is also recommended to obtain androstenedione and testosterone, even in the absence of clinical signs or symptoms.
9.6.2 Fertility Preserving Surgery
Stage 1 disease requires unilateral salpingo-oophorectomy with examination of contralateral ovary. Visible tumors should be removed when reasonable and considered safe. Schneider et al. [45, 46] reported preservation of uterus and the contralateral ovary for children with Stage 2 and 3 disease. Rare lymph node metastasis discourages the need for a routine staging lymphadenectomy, but those which seem to be enlarged at surgery or on imaging should, however, be removed.
Experienced surgical and anesthetic teams are performing minimally invasive surgeries with minimal complications. Care must be taken to avoid intraoperative spillage or rupture, which will upstage the patient. Laparoscopy has the advantage of exploring the abdominopelvic cavity through a small incision, evaluating both ovaries and allowing for fertility-sparing surgery to be performed. The ovary can be removed through one of the port sites after being ligated and transected from its vascular pedicle and its attachment to the uterus. There are also emerging techniques with single port laparoscopy, requiring a single umbilical incision that may improve cosmetic outcomes. Traditional exploratory laparotomy with unilateral salpingo-oophorectomy can also be performed using a vertical incision, which is recommended in the setting of large adnexal masses.
9.6.3 Postoperative Chemotherapy
FIGO Stage 1A with complete resection does not necessitate further treatment or adjuvant chemotherapy. Currently only surveillance with clinical and radiographic monitoring along with tumor markers every 3 months for 2 years is recommended.
FIGO stage 1C with juvenile granulosa cell tumors is treated based on intraoperative rupture and the presence of a low number of mitosis. In such cases, some may argue for observation with frequent imaging, clinical follow-up and serial tumor markers. Standard treatment consists of adjuvant platinum-based chemotherapy after resection, followed by 4 or 6 cycles of BEP for Stage 1C tumors—those with pre-operative rupture and/or malignant ascites (positive washings) or Stages 2/3/4. This regimen is also considered for Stage 1C due to intraoperative rupture with >20 mitosis/10hpf. FIGO Stage 1C or greater with Sertoli-Leydig tumors must be treated with adjuvant platinum-based chemotherapy. Detailed chemotherapy regimens have traditionally been platinum-based: cisplatin/etoposide/ifosfamide (PEI), Bleomycin/Etoposide/Cisplatin (BEP), etoposide/bleomycin, paclitaxel/carboplatin, vincristine/adriamycin/cytoxan, vincristine/cisplatin/bleomycin [44–47].
Regarding those with poorly differentiated tumors, high number of mitosis, and particularly those with Sertoli-Leydig cell tumors, higher intensity chemotherapy regimens are recommended. Sertoli-Leydig tumors are aggressive tumors that carry a high risk of relapse and tumor-related death, versus granulosa cell tumors present in other patients. Stage 4 is rare and carries a poor prognosis, with little evidence to guide therapy [44–47].
9.6.4 Surveillance
Patients should be followed with routine imaging: CT, ultrasound, and MRI during and after therapy. Imaging at 3-month intervals is recommended for the first 3 years after diagnosis, with lessening intervals thereafter. Most recurrences happen within the first 3 years following diagnosis. Juvenile granulosa cell tumors recur within the same time period, however, adult granulosa cell tumors may recur after more than 10 years [44, 46]. Tumors markers such an inhibin B and other such hormonal markers, if elevated at diagnosis, should be measured serially every 3 months during follow-up, after completion of therapy [44, 46, 48].
9.6.5 Survival
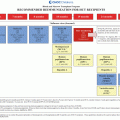
Patients with Stage 1 disease have Event-Free Survival (EFS) of 95.1 % and Overall Survival (OS) of 95.1 % [44, 46]. Those with Stage 2 disease have EFS of 87.5 % and OS of 93.8 % after treatment with standard 6 cycles of cisplatin, bleomycin, and etoposide (BEP). Patients with Stage 3 or Stage 4 disease are usually given higher dose chemotherapy, and may experience greater toxicity without exhibiting any significant difference in survival [44, 46]. Stromal tumors are often confined to one ovary and frequently present with Stage 1 disease, and surgery is the effective management of these tumors, with a favorable prognosis. Patient outcomes are associated with tumor stage and mitotic activity. Tumors with >20 mitosis/10 hpf have an EFS of 0.48, versus those with tumors <20 mitosis per 10 high power field with an EFS of 1.0. Recurrence of Stage 1 ovarian sex cord-stromal tumors is uncommon. Recurrence is most common during the first 2.8 years, usually in the abdominopelvic area or regional lymph nodes. Rare case reports of hematogeneous spread to the chest, liver and bone have been described more commonly with adult granulosa cell tumors [44, 46]. However, the majority of patients will present with a low tumor stage, and therefore will have an excellent prognosis.
9.7 Borderline Tumors
Borderline epithelial tumors are characterized as epithelial tumors with varying degrees of nuclear atypia that lack stromal invasion of the ovary. These tumors are more common than invasive epithelial carcinomas in children, accounting for roughly 31 % of epithelial malignancies. These tumors are very rare with high cure rates. The majority of borderline tumors occur in postmenarchal patients with a median age of 19.7 years old at the time of diagnosis. Borderline tumors, also known as tumors of low malignant potential (LMP), have comprised as much as 20 % of epithelial tumors in children under the age of 18 [50, 51].
9.7.1 Fertility Preserving Surgery
Recommendations include resection of all visible tumor, omental biopsy, and appendectomy if a mucinous tumor is present. This is indicated for the possible presence of a synchronous appendiceal lesion. Cystectomy for Stage 1 borderline tumors is considered appropriate management. Guidelines suggest that there is no benefit to lymph node dissection in clinically normal lymph nodes or random peritoneal biopsies or removing clinically uninvolved areas [50, 51]. Intraoperative frozen section sensitivity has ranged from 62 to 75 %, and therefore necessitates final and thorough pathology review for a correct diagnosis [52, 53]. FIGO recommends unilateral salpingo-oophorectomy or ovarian cystectomy for borderline tumors in patients desiring future fertility. Published data have not suggested that laparotomy is superior to laparoscopy in the hands of a skilled surgeon [50, 51].
9.7.2 Recurrence/Survival
Rate of recurrence after fertility-sparing surgery is as high as 37 %, with the rate increasing after an ovarian cystectomy (12–37.5 %) [50, 51]. Inadequate tumor-free margins after a cystectomy were a risk factor for recurrence [50, 51]. Long-term prognosis is favorable with 5- and 20-year survival of 90 and 80 %, respectively [50–53]. Those patients who underwent cystectomy had a shorter time to recurrence, however, the exact type of surgery does not appear to impact long-term survival, with studies suggesting close follow-up [50, 51, 54]. Long-term recurrences have occurred greater than 10 years from initial surgery in the adult population [50, 51, 54]. Rare case reports have demonstrated invasive recurrence after Stage 1 borderline tumors, and is estimated to be less than 1 % [50, 51, 54]. Patients who under fertility-sparing surgery have excellent reproductive outcomes with fertility rates ranging from 40 to 70 %. The use of infertility treatments is safe for these patients [50–53].
The National Comprehensive Cancer Network guidelines recommend follow-up every 3–6 months for up to 5 years, followed by annual evaluations. Patients should undergo a physical exam which includes a pelvic examination and continued serum markers if elevated prior to surgery (CA-125) [55].
9.8 Epithelial Ovarian Cancer in Adolescents
Epithelial ovarian neoplasia in adolescent girls and young women is very rare, but remains part of the differential diagnosis of any ovarian mass. Studies have shown that epithelial tumors affect roughly 10–28 % of the pediatric and adolescent population, with approximately 65 % of the epithelial tumors occurring in patients over the age of 17 [56]. These are much lower than the 60–80 % reported in the adult population. Tumors most commonly are unilateral and vary in size from 2.5 to 21 cm with mean diameter of 11.7 cm. Epithelial tumors comprise less than 20 % of ovarian tumors in pediatric patients, and rarely occur before menarche [54, 56]. Many authors have suggested that hormonal stimulation is necessary to trigger the development of epithelial ovarian tumors. Specifically in one report, all epithelial tumors in patients under 14 years of age were benign, whereas the 8 malignant epithelial tumors in this series occurred in patients over 15 years old [54]. The histological subtypes that commonly present in childhood are the serous and mucinous tumors. These tumors are further classified into benign, malignant, or borderline (of low malignant potential).
Benign cystadenomas are the most frequent type of epithelial tumor in this population, with serous cystadenomas more prevalent than mucinous ones [54]. Adenocarcinoma in children is a rare phenomenon, with only case reports in the literature. Mortality rates are clearly higher when these carcinomas arise in premenarchal girls. Imaging studies are useful for preoperative assessment to evaluate the presence of ascites and to anticipate the extent of disease. The most commonly used modalities are abdominal and pelvic computerized tomography (CT) or magnetic resonance imaging (MRI). Chest CT may also be performed to evaluate for pleural effusion, pulmonary metastases, and mediastinal lymphadenopathy.
Unlike germ cell tumors, epithelial ovarian tumors frequently cause an elevation in CA-125. Careful follow-up of these patients is required by routine surveillance with imaging and CA-125 levels.
9.8.1 Fertility-Sparing Surgery
Intraoperative adult staging protocols are recommended if there is concern for epithelial ovarian cancer present, with optimal responses to chemotherapy achieved in the setting of minimal disease. Roughly 25 % of adult patients present with tumors confined to the ovary (Stage 1) or tumors beyond the ovary but confined to the pelvis (Stage 2). These patients must be managed with maximal cytoreductive surgery. Systemic chemotherapy may or may not be recommended. When the tumor has metastasized throughout the peritoneal cavity or involves paraaortic or inguinal lymph nodes (Stage 3), or the tumor has spread to more distant sites (Stage 4), the standard of care is surgery followed by systemic chemotherapy. Improved survival rates are noted with the combination of optimal cytoreductive surgery (defined as less than 1 cm in maximum diameter of residual tumor) and platinum-based chemotherapy. Studies have shown that the volume of residual disease after cytoreductive surgery inversely correlates with survival [29, 57]. Many studies have demonstrated an improved response to therapy, less platinum resistance, and improved survival with cytoreduction to <1 mm or no visible disease.
A vertical midline incision is the best approach for staging and primary cytoreductive surgery, and generally pfannensteil incisions should be avoided, as they can potentially limit exposure of the pelvis and abdomen, and prevent access to the peritoneal cavity and upper abdomen. Obtaining any free fluid or washings for cytologic evaluation is recommended. Exploration of all intraabdominal organs and surfaces including the bowel, liver, gallbladder, diaphragms, mesentery, omentum, and the entire peritoneum should be visualized and palpated, with suspicious areas biopsied. If there are no such suspicious areas, multiple biopsies should be obtained from the peritoneum of the cul-de-sac, paracolic gutters, bladder, intestinal mesentery, as well as the diaphragm. Omentectomy should be performed, along with an evaluation of pelvic and periaortic nodes with suspicious nodes sent for frozen section and additional node sampling sent for evaluation to exclude the possibility of Stage 3 disease. A total abdominal hysterectomy and bilateral salpingo-ophorectomy is recommended only after pathology has been confirmed and finalized. Fertility-conserving surgery with unilateral salpingo-oophorectomy is an option, especially for Stage 1A epithelial ovarian cancer. Most surgeons do not routinely biopsy the contralateral ovary if it appears normal. Clinically occult bilateral ovarian involvement has been noted in only 2.5 % of women undergoing staging for ovarian malignancy. Ovarian surgery may impair future fertility, which is the purpose of conservative surgery [57].
Pseudomyxoma peritonei may be present, commonly erupting free mucin deposits into the peritoneal cavity with implants of mucinous epithelium. Pseudomyxoma peritonei has been diagnosed in combination with benign, malignant, and borderline tumors of the ovary. Prognosis relies upon successful removal of all visible mucin, and mucin-producing epithelium [57]. Surgical management consists of removal of the appendix, bilateral ovaries, omentum, and residual mucin. Resection of bowel, diaphragm, peritoneum and mesentery may be required, followed by irrigation, and subsequent chemotherapy.
9.8.2 Adjuvant Chemotherapy
Treatment of early-stage epithelial cancer includes intravenous adjuvant chemotherapy. Standard protocols consist of six cycles of a platinum-based doublet, such as paclitaxel and carboplatin, as this has demonstrated efficacy in the adjuvant therapy of women with advanced-stage epithelial ovarian cancer. Maximal benefit of six cycles has typically been limited to women with serous cancers or stage II disease [57]. It is advisable to individualize the number of treatment cycles based upon patient risk factors and chemotherapy tolerance, with a minimum of three cycles.
9.8.3 Surveillance
Follow-up should include office visits with physical and pelvic exams every 3–6 months for up to 5 years post-treatment, and then annually. Careful follow-up with post-operative imaging and CA-125 levels is critical to detect recurrence or progression of disease. CA-125 levels should continue for every visit if initially elevated, with computed tomography scan, or transvaginal ultrasound in those who underwent fertility-sparing surgery, as clinically-indicated.
9.9 Small Cell Carcinoma
Small cell carcinomas of the ovary are extremely rare, with an incidence of one child or adolescent in a population of 80 million. These tumors are known to be very aggressive with unfavorable prognosis, and may demonstrate paraneoplastic hypercalcemia in 66 % of patients [59]. The tumors are predominately unilateral with a mean diameter of 14.5 cm. Less than 30 % of small cell carcinomas of the ovary develop in patients under 20 years old, and less than 1 % develop in children, with the youngest patient diagnosed at 14 months of age [59]. Patients commonly present with FIGO Stage 2 to Stage 4 disease. The most common presenting symptoms are abdominal pain, constipation, and weight-loss. These are commonly misdiagnosed and require an accurate immunohistochemical work-up for inhibin, which will be positive for sex cord-stromal tumors and negative in small cell carcinomas of the ovary. The staining may demonstrate positivity for parathyroid hormone-related peptide, which explains the etiology of hypercalcemia in these patients. Key management for hypercalcemia includes hydration and diuretic therapy. Favorable prognostic factors include age over 30 years, normal pre-operative calcium, tumor size less than 1 cm, and absence of large cells on pathology [60].
9.9.1 Fertility-Sparing Surgery
Studies have suggested that preservation of the uninvolved ovary and uterus in conjunction with intensive multi-adjuvant chemotherapy does not compromise patients’ overall survival. Due to the highly aggressive nature of the disease, attempts at maintaining reproductive function are only reserved for Stage 1A disease. Aggressive surgical resection is advised with unilateral salpingoophorectomy, lysis of adhesions, omentectomy, appendectomy, bilateral pelvic and periaortic lymphadenectomy, followed by chemotherapy [59]. There is no definitive consensus within the literature, and therefore should be left to the operating surgeon’s discretion.
9.9.2 Treatment
Optimal management includes adjuvant chemotherapy combinations of platinum-based regimen with etoposide, alkylating agents and anthracyclines, with no role for expectant management, even in those with Stage 1A disease. MAKEI protocol recommends adjuvant chemotherapy with 6 cycles of cisplatin, ifosphamide, and doxorubicin, with consolidating high-dose chemotherapy consisting of carboplatin and etoposide in order to reduce the high doses of etoposide leading to secondary leukemia. Some studies have suggested that radiotherapy has a significant therapeutic impact in patients with Stage 1 disease. In patients with macroscopic residual tumor, local deep hyperthermia may be considered [59].
9.9.3 Survival
Patients diagnosed with Stage 1A disease have an event-free survival (EFS) of 33 %; those with 1C have 10 %, and those with Stages 2–4 have an EFS of 6.5 % [59].
9.10 Oncofertility
9.10.1 Ovarian Tissue Cryopreservation
Preservation of fertility is critical to young adult cancer survivors. Numerous survivors will maintain their reproductive potential after the successful completion of treatment, but many still may face problems with infertility several years after the completion of therapy. Infertility is defined as the inability to conceive after 1 year of intercourse without contraception. Rates of permanent infertility and compromised fertility after cancer treatment vary and depend on many factors. Whole pelvic radiation, and numerous chemotherapy regimens containing high dose alkylators put adolescents at risk for acute ovarian failure or premature menopause. The ultimate impact of treatment on reproductive potential depends on the age of the patient at the time of treatment, the type of treatment, the duration, and the total cumulative dose of the treatment administered [61, 62].
9.10.2 Effect of Cancer Regimens on Female Infertility
Females are born with a finite number of primordial follicles, which are depleted through each menstrual cycle leading to maturation of oocytes and subsequent atresia. The chemical toxicity of chemotherapy treatments involves prevention of cell division and adversely affects DNA function within ovarian cells. Each treatment carries a different risk for premature ovarian failure. Higher rates of fertility preservation are seen in women under 30-years-old undergoing treatment with standard protocols. Alkylating agents are as a whole more toxic than the platinum-based therapies. Pelvic irradiaton can have significant effects on the ovary, many of them permanent, even in young girls. Total body irradiation in hematologic malignancies has been shown to affect uterine volume, which is only minimally improved with hormonal therapy [61]. Cranial radiation greater than 35–40 Gy can impair the hypothalamic-pituitary function, and cause hypogonadism through gonadotropin-releasing hormone (GnRH) deficiency [54, 62, 63].
Historically, the assessment of fertility has relied on subjective reports of menstrual history instead of objective parameters of FSH (Follicle-Stimulating Hormone) levels, ovarian volume, antral follicle counts, and AMH (Anti-Mullerian Hormone) levels [48, 64, 65]. Despite resumption of normal menses, there exists a persistent vulnerability for diminished ovarian reserve. Lee et al. found that when objective parameters such as ultrasound and laboratory data were used, reduced follicle counts and alterations were demonstrated in the following hormone levels: FSH, AMH, inhibin B and LH [66]. It should be emphasized that female fertility may be compromised despite the continuation or resumption of seemingly normal cyclic menses and that regular menstruation does not guarantee normal fertility. Any diminished ovarian reserve may result in a lower chance of conception and higher risk of early menopause. Even if women are initially fertile after cancer treatment, the duration of their fertility may ultimately be shortened (see Table 9.2 below) [67].
High risk | >80 % Women develop amenorrhea |
|---|---|
Whole abdominal/ pelvic radiation ≥15 Gy in prepubertal girls and ≥10 Gy in postpubertal girls. | |
External beam radiation that exposes the ovaries | |
Cyclophosphamide 7.5 g/m2 in females under 20 years of age | |
Any alkylating agent (cyclophosphamide, ifosphamide, busulfan, BNU, CCNU) + TBI or whole pelvic irradiation | |
Cranial/ brain radiation ≥40 Gy | |
Intermediate risk | 30–70 % women develop amenorrhea after treatment |
Whole abdominal/pelvic radiation 10 to <15 Gy in prepubertal girls | |
Whole abdominal/pelvic radiation 5–10 Gy in postpubertal girls | |
Spinal radiation ≥25 Gy | |
Low risk | <20 % develop amenorrhea after treatment |
*CMF, CEF, CAF x 6 cycles for women <30 years-of-age | |
Nonalkylating chemotherapy: ABVD, CHOP, COP | |
Anthracycline + cytaribine | |
Low risk | Negligible affects on menses |
Methotrexate + fluorouracil | |
Vincristine | |
Bleomycin/Dactinomycin |
9.10.3 Fertility Preservation Options in Females
9.10.3.1 Embryo Cryopreservation
The most established and effective fertility preservation technique is embryo cryopreservation, which is commonly performed by reproductive endocrinologists and infertility specialists. The process begins with a 10–14 day course of ovarian stimulation from the beginning of the menstrual cycle to produce a large number of mature eggs, or oocytes. The patient then undergoes a minor outpatient surgical procedure to harvest the oocytes through transvaginal aspiration. In vitro fertilization is utilized once the oocytes have been harvested, along with sperm that is fresh or frozen, requiring either the partner or donor’s sperm to create an embryo. These embryos can then be frozen with the use of vitrification, a rapid-freezing method that minimizes crystallization of the embryo to allow for storage, and later, implantation. The cost hovers around $8,000 per cycle and $350 per year for storage fees [67].
9.10.3.2 Oocyte Cryopreservation
Oocyte cryopreservation is still a relatively new technique that requires harvesting and freezing of oocytes or unfertilized eggs after a patient has undergone 10–14 days of ovarian stimulation from the beginning of menstrual cycle. The partner or donor’s sperm is not needed for this process. The oocytes are harvested during an outpatient surgical procedure through transvaginal aspiration, and are then frozen with the use of vitrification. This is an excellent option for patients who may not have a partner, or who are in the pediatric age group. As of 2010, there were over 900 reported deliveries with approximately 60 % of mature oocytes surviving the thaw. The cost is approximately $8,000 per cycle and $350 per year for storage fees [67].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree