In general, FeNa is < 1 % with plasma volume depletion, congestive heart failure, and acute glomerulonephritis but is > 1 % with bilateral ureteric obstruction and ATN; values commonly exceed 3 % with ATN [18]. FeNa validity is degraded by the presampling administration of diuretics [19]. In that case, the FEUN may be more useful as urea excretion is believed to be more dependent on passive forces but is controversial [19–21].
Relatedly, the FEUN may be calculated in a fashion similar to that of FeNa where FEUN is represented by the formula:
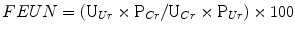
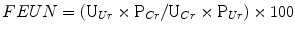
A FEUN < 35 % is consistent with the diagnosis of decreased plasma volume [21, 22]. In some studies, FEUN outperforms FeNa in differentiating between acute renal failure due to prerenal azotemia and that due to ATN.
24-Hour Creatinine Clearance (CrCl24)
This measure is perhaps the most sensitive indicator of renal function in that it relies on a 24-h collection of urine that is kept on ice to retard degradation. The 24-h nature of this test accounts for any diurnal or therapy-driven variations in clearance that would be inherent to a spot or 2-h assessment [23]. This assay also allows the clinician to determine whether the patient’s native renal function exceeds or fails to reach the clearance that can be achieved by intermittent or continuous renal replacement therapy. The major disadvantage is the time required for collection and the need to keep the entire volume of urine on ice.
eGFR
This ubiquitous measure accompanies every laboratory profile that measures Scr and is accompanied by descriptors of age, gender, and race but does not require height and weight [24]. The currently reported eGFR is derived from multiple studies to replace the eGFR derived from the Modified Diet in Renal Disease (MDRD) calculation in patients where the actual GFR exceeds 60 mL/min per 1.73 m2 body surface area [25]. The original MDRD calculation employs age, creatinine, albumin, urea, gender, and ethnicity in its calculation; a 4-variable modification also exists [25]. The eGFR calculation is represented by
![$$ \begin{array}{c} GFR=141\times \min \left( Scr/\kappa, 1\right)\alpha \times \max \Big( Scr/\kappa, 1\Big)-1.209\times 0.993\\ {}\kern0.24em Age\times 1.018\;\left[ if\; female\right]\times 1.159\;\left[ if\; black\right]\end{array} $$](/wp-content/uploads/2017/03/A272285_1_En_4_Chapter_Equc.gif) where Scr is serum creatinine (mg/dL), κ is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 1 [26].
where Scr is serum creatinine (mg/dL), κ is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 1 [26].
![$$ \begin{array}{c} GFR=141\times \min \left( Scr/\kappa, 1\right)\alpha \times \max \Big( Scr/\kappa, 1\Big)-1.209\times 0.993\\ {}\kern0.24em Age\times 1.018\;\left[ if\; female\right]\times 1.159\;\left[ if\; black\right]\end{array} $$](/wp-content/uploads/2017/03/A272285_1_En_4_Chapter_Equc.gif)
This test is primarily used as a screening tool to readily follow the trend in renal function and will be useful over time as the patient ages. Cutoffs for eGFR have been articulated to help categorize the stage of renal failure [3–5]; stages 1 and 2 are applied to renal function estimates when there is a structural abnormality that is present – otherwise a eGFR of 60–89 is not considered abnormal [26]. eGFR is inaccurate in multiple conditions including but not limited to acute renal failure, age < 18, pregnancy, edematous states, severe protein-calorie malnutrition, muscle wasting diseases, critical illness, and following extremity amputation [27, 28].
Urinary Biomarkers
Since Scr and the measures explored above are insensitive and inaccurate in determining early AKI to perhaps enable early therapy that might change the seemingly invariate mortality rate associated with this injury, a more sensitive marker would be ideal in clinical practice. A variety of biomarkers including kidney injury molecule-1 (KIM-1), N-acetyl-β-D-glucosaminidase (NAG), trefoil factor 3, cyanuric acid, cystatin C, monocyte chemotactic peptide-1, netrin-1, and IL-18 have been proposed as sensitive indicators of renal injury or failure. A KIM-1 assay is available but currently remains restricted to research applications. Only urinary neutrophil gelatinase-associated lipocalin (NGAL) has been made commercially available for clinical application [29, 30]. Of note, sepsis-associated-AKI patients have been noted to have higher urinary NGAL levels compared to those with AKI from other causes [31]. In research undertakings, a combination of biomarkers to establish a profile outperforms any single entity in detecting acute kidney injury after cardiac surgery, and current thinking supports establishing a panel-based profile rather than relying on a single marker to identify AKI.
Limitations in Renal Function Assessment
Recall that the kidney has multiple functions that span, in part, regulation of salt and water concentration, blood pressure, red blood cell production, as well as nitrogenous metabolic product and waste clearance. In general, clinicians only regularly assess those related to salt and water clearance, with a lesser assessment (indirectly) of nitrogenous metabolic product and waste handling. Less frequently, in-depth assessments are undertaken (including on rare occasion, renal blood flow), but there is little assessment, if ever, in the clinical arena of hormone function (endocrine, autocrine, or paracrine). Similarly, renal replacement therapy is generally limited to nonhormonal functions as well. Thus, assessment of renal function is limited at best.
Epidemiology of Acute Kidney Injury and Acute Renal Failure
An accurate analysis of the epidemiology of acute kidney injury and acute renal failure is hampered by a wide variety of definitions that describe each of these entities. For example, acute renal failure in many clinical investigations has been defined as a doubling of baseline Scr, an Scr > 2.0, the need for renal replacement therapy (based on clinician determination, not a proscribed protocol), tripling of Scr, as well as a function of changes in urine flow that is not necessarily coupled with a change in Scr. As a result, comparing across studies is difficult at best. Further complicating analysis is the fact that the term AKI is relatively new and many patients who were previously labeled as having ARF actually had Stage III AKI instead [32]. In a related fashion, many terms are used in the literature and describe the same process including acute or chronic renal insufficiency, compromise, or failure. Of course, an acute renal injury may also be a structural injury as a result of trauma. The increased use of CT scans in a wide variety of medical and surgical conditions may also influence the epidemiology of AKI and ARF by increasing the at-risk population to contrast and the well-described radiocontrast nephropathy (RCN) that may follow, especially in elderly patients with concomitant dehydration and diabetes [33]. The incidence of AKI has increased in recent years as has the survival rate of geriatric patients with renal insults [34]. New nephrotoxic medications, including immunosuppressives and chemotherapeutic agents, impact the number of patients who are at risk for and develop AKI or ARF [35–37]. Thus, the epidemiology of these two entities should be anticipated to be in flux, especially as the population ages [38]. Global access to, and delivery of, certain diagnostics and therapeutics may establish a geographically biased epidemiology for AKI and ARF as well. Thus, AKI and ARF may occur with disparate frequency in developed compared to developing nations.
Data on AKI and ARF epidemiology does exist for specific hospital domains, including most commonly the intensive care unit. In a fashion similar to that of sepsis and acute lung injury/acute respiratory distress syndrome, the incidence of AKI that does not require renal replacement therapy (RRT) is estimated to be 2,000–3,000 per million population per year [39]. In contrast, the estimates for AKI that does require RRT are 100-fold higher at 200–300 per million population per year. In order to put these numbers into perspective, 4–5 % of intensive care unit patients receive RRT, and as many as 66 % of intensive care unit patients will develop RIFLE classification-defined AKI [39]. In-hospital mortality strongly correlates with the maximum RIFLE class suffered during that episode of care, as well as with progression through each RIFLE stage of risk, injury, and failure [39, 40]. Despite therapy, RRT-requiring AKI carries a 50–60 % mortality rate with up to one in five sustaining permanent dialysis-dependent renal failures [39].
Certain patient populations may have a higher than population expected risk for AKI, including those suffering from sepsis or injury. In a large cohort of nearly 10,000 injured patients, the crude AKI incidence was 18.1 % with a greater than twofold increased mortality rate; advanced age, female gender, increased number of comorbid illnesses, and a greater illness severity all increased AKI risk [40]. Similarly, in a study of greater than 120,000 patients, 27.8 % of septic patients had a sepsis-related diagnosis; 42.1 % of septic patients developed AKI [41]. Sepsis-associated AKI patients were generally more ill, hypotensive, and tachycardic and demonstrated lower PaO2/FIO2 ratio and greater leukocytosis compared to those with AKI of non-septic etiologies. Increased ICU and hospital mortality as well as ICU length of stay was also observed in those with sepsis-associated AKI across all RIFLE categories [41]. These data have important implications for the elderly as they are well represented in the critically ill and injured patient populations. Specific efforts should be pursued at mitigating known risk factors to reduce the incidence and downstream sequelae of AKI in the elderly after critical injury or illness. In particular, AKI predisposes to chronic kidney disease, and the elderly with reduced GFR appear to be at greater risk for this progression than age-matched counterparts with normal GFR [34].
Etiology of Acute Kidney Injury and Acute Renal Failure
The etiology of AKI is complex and multifactorial [42]. Multiple etiologies for the genesis of AKI have been proposed, including, but not limited to, vasoconstriction, leukostasis, venous hypertension, apoptosis, and a disordered humoral factor milieu including hormones, growth factors, receptors, and intracellular signaling mechanisms. Therefore, multiple etiologies may lead to AKI or ARF. Most AKI appears to be a toxic phenomenon rather than purely a volume-based issue. This observation is easily understood as the RCN that occurs in the well-perfused and volume-loaded patient. Therefore, AKI may also not respond to plasma volume expansion with regard to hastening resolution. Seemingly paradoxically, AKI may be worsened by excess volume loading as the excess salt and water (and likely starch in patients with sepsis) may lead to renal parenchymal edema and distorted organ pressure-volume relationships.
Unsurprisingly, therefore, both intra-abdominal hypertension and the abdominal compartment syndrome are increasingly cited as etiologies for AKI and ARF [43]. Several detailed investigations into these entities have been published for the interested reader [44, 45]. Of note, specific mention is made of intrarenal compartment syndrome that may result from renal parenchymal edema (tissue edema and venous hypertension) that may be only incompletely relieved (tissue edema persists) even after abdominal decompression. Thus, AKI may not dramatically or completely improve despite relief of the abdominal compartment syndrome. It is, however, clear that the renal structural and functional changes detailed above place the elderly patient at increased risk for AKI regardless of cause [46]. Nonetheless, acute kidney injury and acute renal failure all directly impact acid–base homeostasis.
Strong Ions, Acute Kidney Injury, and Acute Renal Failure
While acid–base balance has been traditionally taught using the Henderson-Hasselbalch approach, it is occasionally unwieldly as it is logarithmically based and requires the six “Bostonian rules” to account for chronicity and to provide correction of the derived data [47]. Recognizing that the human body is complex, this scheme works well in the clinical circumstance. An alternative to the imprecision of the Henderson-Hasselbalch approach has been articulated by Peter Stewart in 1983 that is termed the “strong ion” approach [48]. Strong ions are cations and anions dissociated from their ionic partners in an aqueous milieu in the physiologic pH range. This approach equates plasma ionic charge with pH through the influence of charge of water dissociation. A complete exploration of the intricacies of this approach is beyond the scope of this chapter. The interested reader is referred to one of several thorough reviews on this topic [49–52]. Nonetheless, this approach provides a concise framework to both teach acid–base physiology and as a platform from which to prescribe a fluid prescription.
In the strong ion approach, rendering the net plasma charge more positive is an alkalinizing influence, and reducing the net plasma positive charge is acidifying. Therefore, fluids may be categorized based on charge difference relative to human plasma and their anticipated impact on pH. Appropriate fluid selection is aided by understanding the patient’s pre-fluid infusion pH. By way of example, if a patient has preexisting metabolic acidosis that is due to lactate from hypoperfusion, the choice of fluid may be irrelevant as plasma volume expansion should correct perfusion defects and result in lactic acid metabolism. However, if the acidosis is from organ failure, infusing an acidifying solution such as 0.9 % NSS may be maladaptive. Similarly, if the patient is metabolically alkalotic, then an acidifying solution is an intelligent approach and is well embraced in the concept of a chloride-responsive alkalosis; 0.9 % NSS is the most acidifying solution in common use and provides a gross excess of chloride relative to plasma. This approach has been used in a variety of settings including those focused specifically on the geriatric patient with excellent outcomes [53].
The strong ion approach has also been evaluated in terms of outcome prediction. The presence of unmeasured ions, a specific entity that is readily ascertained by that approach, correlates with increased mortality risk in diverse patient populations. These populations include those with major vascular injury, unselected but significantly injured patients, as well as pediatric patients [54–57]. Moreover, the specific fluid selected for resuscitation may drive unmeasured ion generation [58]. Unmeasured ions are known to accompany a host of critical illnesses including injury, renal failure, hepatic failure, and following cardiopulmonary bypass [50, 59–61]. Outcome modeling using this approach has not been specifically undertaken in the elderly but offers a potentially fertile domain for future investigation.
Conclusions
Predictable changes in renal function are to be expected with advancing age. The clinician should be cognizant of these expected changes as they may directly impact the evaluation of renal function, medication dosing, fluid selection, and the management of acute kidney injury and acute renal failure. Recognizing that acute kidney injury is a toxic process and not a preload-dependent phenomenon may reduce the common practice of plasma volume expansion for this increasingly recognized clinical entity. The articulation of renal biomarkers may better enable the bedside clinician to accurately identify elderly patients with a clinically inapparent renal injury and initiate therapy or protective strategies in an earlier time frame than was traditionally possible.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree