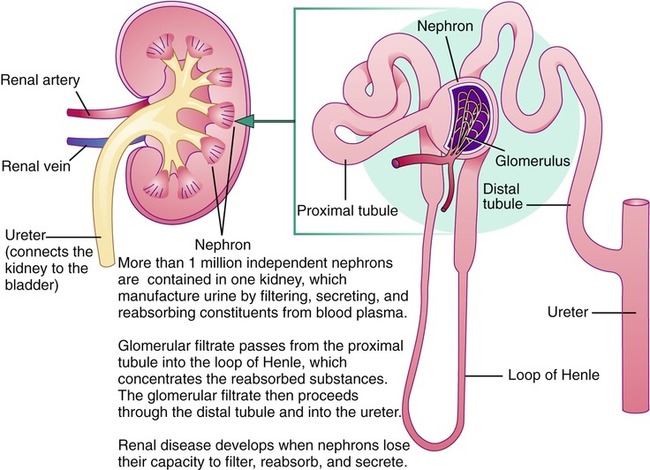
After completing this chapter, you should be able to: • Describe the basic functions of the kidneys. • Identify the clinical symptoms and serum parameters of renal disease. • Identify risk factors for the development of renal disease. • Discuss the principles of nutritional management, including the control of disease and promotion of good nutritional status. • Discuss the role of the nurse and other health care professionals in the management of renal disease. The prevalence of renal (kidney) disease is increasing worldwide, especially in developed countries. In the United States chronic kidney disease (CKD—see later section) has increased in prevalence from just 10 years ago. This is attributed to the increasing incidence of diabetes and hypertension (Coresh and colleagues, 2007). Measures to prevent or reduce obesity in order to reduce the incidence of diabetes and hypertension are critical to the prevention of renal disease (see Chapter 6). Managing renal disease is complex and difficult for the individual with renal disease and the entire health care team. However, renal failure and the need for dialysis may be lessened or even prevented if the person is willing and able to control the diverse, interrelated dietary factors that allow for a normal nutritional balance. Nurses and other health care professionals need to use excellent communication skills to best identify individual needs for self-management of renal disease (see Chapter 1). Registered dietitians are now able to be providers recognized by Medicare (a health program for older adults or those with chronic illnesses) for predialysis renal disease and for diabetes mellitus. The kidneys play a key role in the maintenance of metabolism and hormonal balance. The kidneys convert the inactive form of vitamin D from foods into the active form. They also produce the enzyme renin, which affects systemic blood pressure, and the hormone erythropoietin (EPO), which stimulates red blood cell production by the bone marrow. Figure 9-1 illustrates the composition of the kidneys. The most widely known function of the kidneys is to filter body wastes, including urea, drugs, and toxins. This filtering process occurs in the nephrons, of which there are more than 1 million (see Figure 9-1). With renal disease, medications need to be adjusted to reflect diminished clearance through the kidneys. Persons on diabetes medications may develop hypoglycemia and need to lower the dose of insulin or oral hypoglycemic agents. Health care professionals should be alert to signs and symptoms of various medication toxicities found with reduced renal clearance. The general criteria for diagnosing renal disease center on the functions of the normal kidney. Given that kidneys excrete excess nitrogen, protein, electrolytes, water, and other substances, tests for abnormal levels of these constituents provide an indication of whether renal disease is present and if so, how severe it is. Renal disease progresses along a continuum based on the rate kidneys filter (Box 9-1). Clinical manifestations of renal disease include hematuria (blood in the urine); albuminuria (albumin in the urine); azotemia (nitrogen in the blood); hypertension; edema; hypoalbuminemia (low level of serum albumin; caused by loss of protein in the urine with renal disease); hyperlipidemia; and proteinuria (protein in the urine). A lack of urinary excretion (anuria) and decreased urinary output (oliguria—reduced production of urine to less than 500 mL/24 hours) suggest either significant loss of nephron function or obstruction that can lead to irreversible renal damage. Specific serum indicators routinely used for assessing the degree of renal failure and response to dietary control are the blood urea nitrogen (BUN) level and creatinine (a nitrogenous compound formed in muscle). An elevated BUN level typically occurs before an increased level of creatinine but can be altered based on hydration status. For this reason nephrologists use creatinine levels, in part, to assess renal function. Other serum values are used to assess renal function, including albumin, potassium, phosphorus, sodium, and calcium determinations. All laboratory values are susceptible to error in measurement and interpretation for a variety of reasons. It is because of this that a referral to a nephrologist (a physician specializing in kidney disease) is advised when kidney function reaches stage 4 or 5 (see Box 9-1). One test used to determine renal functioning is the 24-hour urine collection test. A less-reliable version of the 24-hour urine test, but with similar assessment of renal functioning, is the spot urine protein-creatinine ratio. This allows the measurement of glomerular filtration rate (GFR) and gives an indication of how rapidly the kidneys are excreting wastes. A normal GFR is 125 mL/min. A GFR less than 60 mL/min is equated with renal insufficiency, also called chronic kidney disease (CKD; stage 3), and testing for common complications of renal disease is advised. A GFR less than 10 to 15 mL/min (stage 5) is associated with uremia and may involve dialysis treatment (see later section). Stages of kidney disease are based on GFR. The five stages are shown in Box 9-1. Concerning screening and early diagnosis of CKD, serum creatinine level alone is an inadequate parameter for the evaluation of renal function. Proteinuria is both an indicator of renal damage as well as a progression factor for ongoing loss of renal function. Reduction of proteinuria toward levels less than 0.5 g/day is optimal to help prevent decline in renal function and help prevent cardiovascular disease (Brandenburg and Floege, 2006). Predictors of the final point of renal function, called end-stage renal disease (ESRD), include proteinuria as well as reduced GFR less than 10 to 15 mL/min. Other factors associated with ESRD include age, and other risk factors found with CKD include cigarette smoking, hypertension, low high-density–lipoprotein cholesterol (HDL-C) level, and elevated fasting glucose level (Ishani and colleagues, 2006). Ten areas have been identified as important for optimal care of the persons with CKD: • Hypertension (see Chapter 7 for Dietary Approaches to Stop Hypertension [DASH] diet) • Lipid control (see Chapter 7) • Use of a β-blocker following myocardial infarction • Use of ACE inhibitors or angiotensin II receptor blockers • Diet and weight control (see Chapter 6) Hypertension affects one out of four adults in the United States and is part of the metabolic syndrome. Elevated blood pressure is the most significant risk factor for developing CKD. A population at high risk for hypertension and renal disease with proteinuria is African Americans (Lea and colleagues, 2008) Tight blood pressure (less than or equal to 125/75) control has been shown to reduce protein loss in the urine and to delay progression of renal disease (Barri, 2008). In males, albuminuria (another form of protein loss in the urine) was found to be a strong independent predictor for renal function decline (Halbesma and colleagues, 2008). In nondiabetic kidney disease, lower blood pressure slows the decline in GFR (Sarnak and colleagues, 2005). Other factors with risk of CKD include factors found with the metabolic syndrome: Inflammation is now recognized as being involved in the metabolic syndrome. Reduction of inflammation is important to help prevent or manage renal disease. An elevated serum C-reactive protein (CRP) level is strongly associated with morbidity and mortality with CKD in both predialysis and dialysis patients. Elevated levels of CRP are associated with other indicators of inflammation and with reduced GFR (Razeghi and colleagues, 2008). 1. Inflammation or glomerulonephritis 2. Loss of protein caused by damage to the glomerular barrier, which leads to the nephrotic syndrome 3. Damage or scarring to the nephrons, or glomerulosclerosis, which includes diabetic nephropathy as described later In general, the condition of glomerulonephritis, or nephritis, can be temporary or chronic. It is a result of inflammation or damage to the glomeruli (each glomerulus serves as a tiny filter contained within each nephron; see Figure 9-1). The nephritic syndrome commonly follows a streptococcal infection and usually lasts for only a short time, allowing for complete recovery. Symptoms can include hematuria caused by inflammation of the nephrons, mild loss of kidney function, and salt-sensitive hypertension induced by proteinuria and nephritis. Nephritis can develop into the chronic nephrotic syndrome or ESRD. There are a variety of causes of the damage other than infections, such as autoimmune disorders like lupus. There have been case reports of renal dysfunction, including acute interstitial nephritis, associated with use of creatine supplements by athletes (Thorsteinsdottir, Grande, and Garovic, 2006). Warning signs of damage caused by chronic nephritis include hypertension, edema, changes in urine color, nausea and vomiting, and headaches. Diagnosis is often confirmed with a biopsy of kidney tissue. Nephrotic syndrome is a set of symptoms including proteinuria, edema, and hyperlipidemia. Protein excretion is considered normal up to 150 mg (0.15 g) daily. The nephrotic syndrome is associated with loss of several grams of protein daily, resulting in impaired nutritional status. Greater excretion values are found with renal insufficiency, as well as with diabetes, hypertension, and other causes such as pregnancy. Reduced levels of branched-chain amino acids (see Chapter 2) are recognized to occur with CKD. This is, in part, related to renal changes in amino acid metabolism. The nephrotic syndrome is caused by increased permeability of the glomerular capillary wall for proteins (see Figure 9-1). One form of protein lost in the urine is albumin, the condition being called microalbuminuria, or a higher rate of protein lost in the urine (greater than 200 mcg/min) is referred to as macroalbuminuria. The loss of protein in the urine in turn causes a decreased serum albumin level. Albumin less than 3.0 g/dL is found with peripheral edema, ascites (see Chapter 4), and anasarca (generalized massive edema). With low levels of protein, fluid leakage into the interstitial space occurs; it also causes sodium retention. Ascites further inhibits food intake because of a feeling of fullness, exacerbating concerns about poor protein status. Issues of protein loss are multiple. Several vitamin D–binding proteins result in depletion of activated vitamin D metabolites, which promotes the development of osteomalacia (softening of the bones). The loss of lipid carrier proteins results in hyperlipidemia. Blood clotting is enhanced because of loss of protein related to anticlotting factors. This results in increased risk of thrombosis (see Chapter 7). Inflammation can be found with the nephrotic syndrome. Corticosteroids are often the standard first-line treatment of the nephrotic syndrome, in order to reduce inflammation. Various antiinflammatory agents are being researched as alternatives to steroid use. The elevated serum ferritin level found with proteinuria and the nephrotic syndrome is likely part of the inflammatory state (Branten and colleagues, 2004). The broad condition of glomerulosclerosis is often referred to as nephrosclerosis and is related to the many causes of scarring of the glomeruli contained within the nephrons. Chronic hyperhomocysteinemia can cause injury in the kidney, and increased intake of folate and other B vitamins (B1, B2, B6, and B12) is advised. Creatinine clearance was found to decline with elevated plasma homocysteine concentration (Kumagai and colleagues, 2002). A variety of toxic substances, medications, and painkillers are known to cause damage to the kidneys. Diabetic nephropathy is related to sclerosis (scarring) and is a major factor in the development of ESRD. The earliest clinical evidence of diabetic nephropathy is microalbuminuria. Microalbuminuria is associated with prediabetes, most likely because of the higher insulin resistance (Suzuki and colleagues, 2004). Approximately one third of individuals with both type 2 diabetes and microalbuminuria develop nephropathy within a 10-year period. Newly diagnosed type 2 diabetes may already be found with nephropathy because of a possible delay in diagnosis of this form of diabetes. Therefore anyone newly diagnosed with type 2 diabetes is advised to have renal function assessed; assessments should be made every 5 years for either type 1 or type 2 diabetes. Persons with diabetes should be regularly monitored for microalbuminuria and not gross proteinuria to best detect kidney dysfunction (Al-Homrany and Abdelmoneim, 2004). As discussed in Chapter 8, the Diabetes Control and Complications Trial (DCCT) found that normalizing blood glucose levels to an average of less than 155 mg/dL or hemoglobin A1c (Hgb A1c) under 7.2% was found to reduce kidney disease by about half. It is hoped that achieving even more-normal blood glucose levels may further reduce the development of nephropathy and ESRD with the goal A1c being in the 6% range. If there is no risk of hypoglycemia, a goal for the A1c in the 5% range is appropriate as achieved by medical nutrition therapy (MNT) (see Chapter 8). The glycosylation of renal structures may be the cause of nephropathy, as found with uncontrolled diabetes. Although there generally seems to be a protection from renal disease for women, as compared with men, this is not the case with diabetic nephropathy. However, management of estrogen levels in menopausal women may help reduce risk of renal disease, especially diabetic nephropathy (Maric and Sullivan, 2008). In one case study, acute kidney injury from oxalate nephropathy has been associated with increased fat malabsorption and frequent loose oily stools due to use of orlistat for weight loss. Discontinuation of orlistat allowed steady improvement in renal function (Singh and colleagues, 2007). This has potential ramifications with bariatric weight loss surgery that leads to fat malabsorption (see Chapter 6 and later section on kidney stones). Avoidance of excess vitamin C supplementation also is important to prevent high oxalate levels. Contrast-induced nephropathy continues to be a common complication of coronary angiography. The administration of intravenous saline or possibly sodium bicarbonate and the antioxidant acetylcysteine may reduce the risk of contrast-induced nephropathy (Iyisoy and colleagues, 2008). An immune factor called immune globulin A (IgA) has been linked with one form of nephropathy. In a case study involving IgA nephropathy, unrecognized celiac disease was found and both conditions improved with a gluten-free diet (La Villa and colleagues, 2003). Omega-3 fatty acids have been found beneficial, as evidenced with the purified form called Omacor, lowering proteinuria (Hogg and colleagues, 2006). A new term is now advised to describe the broader clinical syndrome of disordered mineral and bone metabolism: CKD-mineral and bone disorder (CKD-MBD). CKD-MBD includes an abnormality of one or more of the following: altered levels of calcium, phosphorus, PTH, or vitamin D metabolism; bone changes; and calcification of the vascular system or other soft-tissue calcification (Moe and colleagues, 2007). Because the nephritic syndrome is generally of a self-limiting nature, MNT is primarily aimed at maintaining nutritional well-being until recovery has occurred. Mild restrictions of protein and potassium may be indicated but should be based on serum laboratory values. Edema may necessitate a moderate salt restriction to help preserve renal function with glomerulonephritis, especially if there is high blood pressure (Box 9-2). A beneficial effect of fish oil on survival of individuals with lupus nephritis has been observed and may be a result of decreased atherosclerosis (Muthukumar and colleagues, 2003).
Renal Disease
INTRODUCTION
WHAT ARE THE FUNCTIONS OF THE KIDNEYS?
WHAT ARE DIAGNOSTIC PARAMETERS OF RENAL DISEASE?
HOW IS RENAL DISEASE SCREENED AND ITS COMPLICATIONS PREVENTED?
WHAT ARE SOME TYPES AND PHYSIOLOGY OF RENAL DISORDERS?
GLOMERULONEPHRITIS
NEPHROTIC SYNDROME
GLOMERULOSCLEROSIS
DIABETIC NEPHROPATHY
IGA NEPHROPATHY
CHRONIC KIDNEY DISEASE
WHAT IS MEDICAL NUTRITION THERAPY IN RENAL DISEASE?
NEPHRITIC SYNDROME

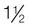 lb
lb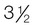 oz
oz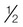 c
c c
c c
c

