Renal Disease
1 DaVita Healthcare Partners, Philadelphia, PA
2 Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA
Acute Kidney Injury
Acute kidney injury (AKI) is the current name for acute renal failure. It is still characterized by a sudden decline in the glomerular filtration rate (GFR) of the kidney due to insults such as infection, exogenous nephrotoxins, trauma, dehydration, and shock resulting in ischemia, but is now staged with the hope that early intervention will result in better outcomes. (See Table 10-1). Patients with AKI are at high risk for malnutrition because of underlying illnesses, recent surgical procedures, or trauma, all of which place them in a catabolic, pro-oxidative, and proinflammatory state. In AKI precipitated by major trauma, critical illness, or sepsis, patients frequently undergo metabolic changes that accelerate degradation of protein and amino acids and result in the loss of lean body mass. The dramatic effects of this catabolic state include poor wound healing, increased infection, increased hospitalization, and higher mortality rates.
Table 10-1 Criteria for Stages of Acute Kidney Injury (AKI)
Source: Hark L, Ashton K, Deen D. The Nurse Practitioner’s Guide to Nutrition, 2nd Edn. 2012: John Wiley & Sons, with permission.
| Stage | Creatinine | Urine Output |
|---|---|---|
| 1 | Serum creatinine increased by 0.3 mg/dL or 1.5–2 times the baseline value | <0.5 ml/kg/h for >6 h |
| 2 | Serum creatinine increased 2–3 times the baseline value | <0.5 ml/kg/h for >12 h |
| 3 | Serum creatinine increased >3 times the baseline value or serum creatinine ≥4.0 mg/dL with an acute increase of 0.5 mg/dL | <0.3 ml/kg/h for 24 h or anuria (no urine ouput) for 12 h |
Medical Nutrition Therapy for Acute Kidney Injury
Since malnutrition is so often seen in patients with AKI and is known to be an independent risk factor contributing to increased mortality, implementation of medical nutrition therapy is very important. Decisions on when to implement and how aggressive medical nutrition therapy should be depend on the patient’s nutritional status and catabolic rate, the phase of AKI, the amount of urine output, and clinical indications such as uremia or volume overload requiring dialysis or continuous renal replacement therapy (CRRT). Thus, medical nutrition therapy for the patient with AKI must be highly individualized and the goals are to:
- preserve protein stores
- hinder skin breakdown
- prevent nutritional deficiencies until renal function returns, while maintaining fluid, electrolyte, and acid-base homeostasis.
Protein
Restricting protein intake to 0.8 g/kg per day may be indicated only for patients with AKI whose GFR falls to less than 10 mL/min and who are not catabolic or on any form of dialysis or CRRT. All forms of dialysis and CRRT contribute to protein losses. The protein intake of patients who are receiving hemodialysis (HD) should be at least 1.2 g/kg per day and patients receiving peritoneal dialysis (although infrequently used with AKI) are encouraged to ingest 1.2 to 1.3 g/kg of protein each day. Severely catabolic patients with AKI may have even higher protein needs and require CRRT or aggressive dialytic therapy to allow for sufficient protein intake.
Energy
Caloric requirements for patients with AKI vary depending on the degree of hypermetabolism. Usual recommendations are 35 kcal/kg per day, however, needs may actually be closer to only 20 to 30 kcal/kg per day, especially when total parenteral nutrition (TPN) is utilized. The most accurate determination of caloric requirements is by indirect calorimetry, but equipment to do so may be limited by cost and availability. Complications from slightly underfeeding are not as harmful as overfeeding patients with a high dextrose load, which can cause hyperglycemia, triglyceridemia, and CO2 retention in patients with respiratory disease. Calories from the dextrose utilized in dialysate with peritoneal dialysis (PD), as well as in replacement fluids with CRRT, must be considered. Now, however, replacement fluids generally utilized with CRRT only have physiologic amounts of dextrose and use bicarbonate rather than lactate, thus do not contribute a significant source of calories. Also, PD is rarely utilized for patients with AKI.
Patients who have adequate gastrointestinal tract function but cannot tolerate food by mouth because of mechanical ventilation, altered mental status, anorexia, nausea, or poor adherence, should receive nourishment by enteral tube feeding (Chapter 12). Those with a dysfunctional GI tract require parenteral nutrition (Chapter 13). Peripheral insulin resistance may cause hyperglycemia in catabolic patients with AKI, therefore blood glucose levels should be closely monitored. Insulin may be required, especially with the use of parenteral nutrition. Also, there may be alterations in lipid metabolism in patients with AKI. Lipids are not precluded in parenteral feedings, but triglyceride levels must also be monitored closely.
Vitamins and Minerals
Vitamin and mineral requirements for patients with AKI vary depending on the patient’s nutritional status and whether they are receiving dialysis or CRRT. Serum electrolytes must be closely monitored in all patients with acute kidney injury (AKI). Initially, serum potassium and phosphate are likely to be elevated and serum sodium lowered in non-dialyzed patients who are oliguric (urine output <400 mL/day). Patients with acute intrinsic renal failure (usually defined as acute tubular necrosis – the major cause of AKI) may experience salt and water overload during the oliguric phase and salt and water depletion during the diuretic or recovery phase of the disease when urine output can exceed 2 to 3 liters per day. In the recovery phase, sodium, potassium, and fluid may need to be replaced to offset urinary losses. Oliguric or anuric patients receiving HD usually require a sodium restriction of 2 to 3 g/day and a potassium restriction of 2 to 3 g/day. Those undergoing PD, frequent HD (more than three times per week), and some forms of CRRT generally have more liberal sodium and potassium requirements. Patients with AKI undergoing any form of dialysis or CRRT should receive supplemental water-soluble vitamins above the Recommended Dietary Allowances (RDA) to compensate for losses with these treatments. For those receiving total parenteral nutrition (TPN), the standard multivitamin dose should be feasible, especially when TPN is short-term.
Fluid
Daily fluid intake for oliguric patients should equal urine output plus approximately 500 mL to replace insensible losses; fluid needs increase if the patient has a fever. Most anuric patients can tolerate approximately 1000 mL/day with HD three times per week. These restrictions may be liberalized in patients receiving continuous or daily peritoneal dialysis, CRRT, or hemodialysis more frequently than three times per week.
Continuous Renal Replacement Therapy
Continuous arteriovenous hemofiltration (CAVH) utilizes catheters that are placed into a large artery and vein (often the femoral artery and vein). The arterial blood flows through a small filtering device with a large porous membrane where plasma is filtered of water, minerals, and uremic toxins, and albumin and blood products return to the vascular space through the vein. This form of therapy removes large volumes of essentially albumin-free plasmanate, leaving water and electrolytes in a concentration equal to normal serum levels. It is often used for patients who are very volume overloaded and cannot tolerate standard HD due to very low blood pressure. Parenteral nutrition can be combined with CAVH to provide intravenous nutrition while controlling sodium and water balance and removing small amounts of metabolic waste products that accumulate in renal failure.
Continuous arteriovenous hemodiafiltration (CAVHD) combines HD and hemofiltration simultaneously and removes larger amounts of solutes as well as large volumes of fluid. CAVH and CAVHD use systemic arterial blood flows; other forms of CRRT including continuous venovenous hemofiltration (CVVH) and continuous venovenous hemodiafiltration (CVVHD) use a pumping machine that may result in less erratic blood flows and ultrafiltration rates.
Chronic Kidney Disease (Pre-Dialysis)
In 2002, the National Kidney Foundation (NKF) published clinical care guidelines for those with chronic kidney disease (CKD). These guidelines help determine the stage of kidney disease based on kidney damage and/or level of glomerular filtration rate (GFR) as shown in Table 10-2. Stage 1 includes kidney damage (e.g. proteinuria) with normal GFR or a GFR of 90 or above. Stage 2 includes mild kidney damage and a GFR of 60 to 89. Stage 3 includes a moderate decrease in GFR to 30 to 59. In Stage 4 the GFR is 15 to 29 and in Stage 5 the GFR is less than 15. Medical nutrition therapy goals for patients with CKD Stages 1 to 4 prior to dialysis or renal transplantation are to retard the progression of CKD while providing adequate calories to maintain or achieve ideal body weight, and to prevent or alleviate the symptoms of uremia and restore biochemical, calcium/phosphorus, vitamin, and iron balance.
Table 10-2 Clinical Care Guidelines for Chronic Kidney Disease
Source: Hark L, Ashton K, Deen D. The Nurse Practitioner’s Guide to Nutrition, 2nd Edn. 2012: John Wiley & Sons, with permission.
| Stage of CKD | Level of Kidney Damage | GFR (mL/min) |
|---|---|---|
| 1 | Kidney damage (e.g., proteinuria) | Normal or ≥90 |
| 2 | Mild kidney damage | 60–89 |
| 3 | Moderate decrease in GFR | 30–59 |
| 4 | More severe decrease in GFR | 15–29 |
| 5 | Severe decrease in GFR | <15 |
Medical Nutrition Therapy for CKD
Protein
In CKD, as the GFR and excretion of nitrogenous wastes decline, it is necessary to control the level of protein intake while continuing to maintain a positive nitrogen balance. Protein restriction can minimize the symptoms of uremic toxicity by reducing the production of nitrogenous wastes in the blood. Some evidence also suggests that protein restriction early in the course of CKD due to glomerular damage may slow the progression of the disease and delay the need to initiate dialysis therapy. The generally accepted level of protein restriction for patients with CKD stages 1 to 3 is 0.75 g/kg per day, which is approximately what the DRIs recommend for normal, healthy adults. This is actually a restriction for most individuals, as the American diet is generally much greater in protein content. For stages 4 and 5 (GFR <25 mL/minute), 0.6 g/kg per day (using an adjusted body weight if the patient is obese) is suggested, only if feasible to meet overall nutritional needs.
Approximately 50 percent of high biological value protein (HBV) is usually encouraged to ensure that essential amino acid requirements are met. The biological value of a dietary protein is determined by its constituent amino acids, with the highest value given to proteins that contain all essential amino acids, such as eggs, meats, and other animal proteins. It has also been shown, however, that carefully planned low-protein vegetarian diets containing soy and plant-based protein may reduce proteinuria, improve serum protein levels, and retard the progression of CKD as compared to animal proteins. Additional increased protein needs due to catabolism from use of glucocorticoid (steroid) therapy or recent surgery as well as acute illness with decreased oral intakes may contraindicate limiting dietary protein.
Energy
The recommendations for adequate energy intake for individuals with CKD not yet on dialysis are generally 35 kcal/kg per day to maintain body weight and allow for effective protein utilization. It has been recommended that 30 kcal/kg per day be used for those older than 60 years of age due to a more sedentary lifestyle. Calories from complex and simple carbohydrates must be included in the diet to provide adequate energy to prevent weight loss. Low protein food products are available and can improve overall caloric intake while minimizing protein content, but the expense, taste, and availability must be considered.
Lipids
Additional fat, in the form of monounsaturated and polyunsaturated fats, may also be recommended to provide adequate calories for patients with CKD. Since dyslipidemia is prevalent in patients with CKD, lipid levels should be monitored, and an effort made to keep total cholesterol, LDL-C, HDL-C, and triglyceride levels within normal limits (Chapter 6). Pharmacologic therapy may be needed to manage lipid levels, as some studies utilizing statins have shown cardiovascular risk reduction for patients with CKD stages 2 to 3.
Sodium
As renal failure progresses to a GFR of about 10 percent of normal, renal sodium excretion subsequently falls. Sodium intake may have to be limited to prevent sodium retention, generalized edema, hypertension, and/or congestive heart failure, especially in the advanced stages of CKD when excretion diminishes. The NKF/KDOQI Clinical Practice Guidelines on Hypertension and Antihypertensives recommend a sodium intake of less than 2.4 g/day unless a sodium wasting disease is present or medications causing sodium loss are prescribed.
Measuring urinary sodium in a 24-hour urine collection may be helpful in determining how much sodium is being excreted. Urinary sodium is reported in milliequivalents (mEq), making it necessary to convert from milligrams to milliequivalents to determine how many milliequivalents of sodium are associated with any given diet.
Table 10-3 Foods With High Sodium Content
Source: Lisa Hark, PhD, RD. Adapted with permission.
| Bacon |
| Barbecue sauce |
| Bouillon cubes* |
| Canned seafood* |
| Cheeses, processed |
| Chinese food |
| Cold cuts |
| Corned beef |
| Corn chips |
| Crackers* |
| Dried beef |
| “Fast Foods” |
| Frozen dinners (unless of a healthy variety). |
| Gravy, canned or packaged |
| Ham |
| Hotdogs |
| Meat tenderizers |
| Nuts, salted* |
| Olives |
| Packaged or prepared casserole dishes |
| Popcorn |
| Pickles |
| Potato chips, pretzels* |
| Relish |
| Salt pork |
| Sauerkraut |
| Sausages |
| Scrapple |
| Smoked meats or fish |
| Soy sauce |
| Steak sauce |
| Soups, canned* & dried mixes |
| Tomato juice* |
| Tomato sauce |
| Vegetable juice* |
| Worcestershire sauce |
Some of the above foods may be acceptable if allowed in small servings.
*These items may be purchased “salt-free” or “low sodium” in many grocery stores. If following a low potassium diet, be sure to read labels as potassium chloride may substituted for sodium chloride; then these specific items should not be used.
Potassium
The kidney usually handles potassium efficiently until the GFR is significantly reduced (<10 mL/min). Thus, a dietary potassium restriction may be necessary only during the latter stages of CKD. Exceptions include renal diseases such as diabetic nephropathy, in which aldosterone deficiency develops and potassium excretion declines. Use of an angiotensin-converting enzyme (ACE) inhibitor to control blood pressure in some individuals may also require a mild-to-moderate potassium restriction, even with good urine output. ACE inhibitors suppress the renin-angiotensin system, resulting in decreased aldosterone levels and subsequent elevations in serum potassium levels. Angiotensin receptor antagonists used to control hypertension can also cause hyperkalemia, though the likelihood is probably lower than with ACE inhibitors. When serum potassium levels are consistently greater than 5.0 mEq/L, a potassium-restricted diet of 2 to 3 g/day (51 to 77 mEq/day) should be initiated (Table 10-4).
Table 10-4 Foods With High and Low-To-Medium Potassium Content
Source: Lisa Hark, PhD, RD. Used with permission.
| High-Potassium Vegetables | High-Potassium Fruits and Juices |
| Artichokes Beans (navy, lentil, kidney, pinto) Broccoli Brussels sprouts Carrots, raw French fries, Greens Lima beans Parsnips Potato, baked and chips Pumpkin Spinach Sweet potato Tomato Winter squash (butternut, acorn) Tomato juice Vegetable juices | Apricots Avocados Bananas Cantaloupes Dates Figs Honeydew melons Mangos Nectarines Oranges, orange juice Papayas Prunes Raisins Rhubarb Watermelon (if more than one cup chunks) Apricot nectar Prune juice |
| Other high-potassium foods | |
| Milk (more than 4 to 8 ounces/day) Chocolate Nuts Bran cereal | Salt substitutes (containing KCL) Molasses Potato chips |
| Low-to-medium potassium vegetables* | Low-to-medium potassium fruits and juices* |
| Asparagus Beets Cabbage Carrots, cooked Cauliflower Celery Corn Cucumber Eggplant Green beans Green peppers Kale Lettuce Okra Onions Peas Potato (only when double-boiled) Radishes Wax beans Zucchini | Apples, apple juice Applesauce Blueberries Cherries Cranberries, cranberry juice Fruit cocktail Grapefruits, grapefruit juice (only 4 ounces/day) Grapes, grape juice Lemons Limes Peaches, fresh (small) Pears, fresh (small), pear nectar Pineapples, pineapple juice (only 4 ounces/day) Plums Raspberries (1 cup) Strawberries (1 cup) Tangerines |
*Note that even low-to-medium potassium foods must be consumed in limited amounts daily.
Calcium, Phosphorus, Parathyroid Hormone, and Vitamin D
Mineral-Bone-Disorder (MBD) describes the clinical syndrome resulting from abnormal mineral bone metabolism which occurs with CKD. Renal osteodystrophy refers to only the complex bone lesions present in the majority of patients with CKD, and includes osteitis fibrosa and osteomalacia, which are associated with this disorder. Restriction of dietary phosphorus has been shown to prevent the development of secondary hyperparathyroidism, which is frequently seen in patients with CKD. Also, in the past decade increased vascular and soft tissue calcifications have been seen in this population, believed to be related to calcium/phosphorus metabolism and treatment to maintain proper balance of these minerals. As a result, the NKF/KDOQI Clinical Practice Guidelines for Bone Metabolism and Disease recommend a phosphorus restriction of 800 to 1000 mg/day for individuals with CKD stages 3 and 4 when the serum phosphorus level is greater than 4.6 mg/dL (Table 10-5). With a protein-restricted diet, this is usually feasible, as animal protein-based foods are also high in phosphorus content. If dairy products are avoided in a vegetable-based low-protein diet utilizing soy products, this level of phosphorus restriction is also feasible.
Table 10-5 Phosphorus Content of Selected Foods
Source: Hark L, Ashton K, Deen D. The Nurse Practitioner’s Guide to Nutrition, 2nd Edn. 2012: John Wiley & Sons, with permission.
| Foods | Portion Size | Phosphorus Content (mg) |
|---|---|---|
| Dairy | ||
| Cheese, cheddar | 1 ounces | 145 |
| Cheese, cream | 1 Tbsp. | 15 |
| Frozen yogurt | ½ cup | 95–100 |
| Half-and-half | ½ cup | 110 |
| Ice cream | ½ cup | 70–100 |
| Milk (whole, low-fat, skim) | 8 ounces | 220–230 |
| Pudding (vanilla/chocolate dry mix regular made with 2% milk) | ½ cup | 115–135 |
| Pudding (chocolate dry mix instant made with 2% milk) | ½ cup | 350 |
| Pudding, (vanilla/chocolate/ tapioca/ rice-ready-to-eat) | ½ cup | 45–75 |
| Yogurt (all kinds) | 8 ounces | 215–350 |
| Protein foods | ||
| Beef, cooked | 3 ounces | 150–200 |
| Eggs, whole | 1 large | 95 |
| Liver, Beef (panfried) | 3 ounces | 410 |
| Peanut butter | 1 Tbsp. | 55 |
| Sardines, Atlantic, canned in oil | 3 ounces | 415 |
| Tuna | 3 ounces | 140–265 |
| Vegetables | ||
| Baked beans and pork and beans | ½ cup | 95–150 |
| Dried beans | ½ cup | 130 |
| Chickpeas | ½ cup | 110–140 |
| Lentils, boiled | ½ cup | 180 |
| Soybeans, green boiled | ½ cup | 140 |
| Soybeans, mature boiled | ½ cup | 210 |
| Bread and cereals | ||
| Barley, pearled cooked | 1 cup | 85 |
| Bread, white | 1 slice | 25 |
| Breads whole grain | 1 slice | 60 |
| Cornbread (from mix) | 1 piece | 225 |
| Raisin Bran | 1 cup | 225 |
| Miscellaneous | ||
| Chocolate | 1 ounce | 70 |
| Nuts, mixed, dry | 1 ounce | 125 |
| Peanuts, dry roasted | 1 ounce | 100 |
| Beverages | ||
| Beer | 12 ounces | 50 |
| Coffee, brewed | 6 ounces | 5 |
| Colas | 12 ounces | 60 |
Note that inorganic phosphate contained in beverages and other food products are absorbed 100%, even if total phosphorus content does not seem high. Check labels for any ingredient containing the letters “phos” and avoid these products if following a low phosphorus diet.
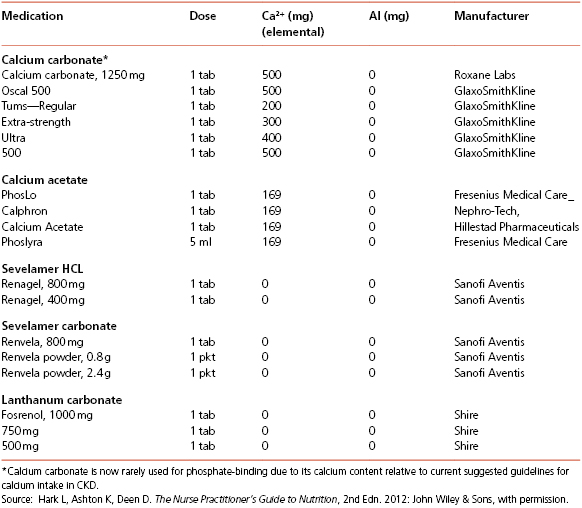
A “phosphate binder”, which may be prescribed with meals, interferes with the absorption of phosphate in the small intestine while maintaining serum phosphate levels within normal range. Calcium acetate, sevelamer hydrochloride or sevelamer carbonate (non-absorbed phosphate-binding polymers without calcium or aluminum), and lanthanum carbonate have also been utilized (Table 10-6). These medications are used “off-label” for CKD patients not yet on HD. Sometimes, a combination of sevelamer and calcium acetate is used to provide phosphate binding without adding significant calcium. Serum calcium levels may not decrease until the GFR is less than 30 mL/minute, thus initially eliminating any need for specific calcium supplementation until later stages of CKD. Since foods rich in calcium (primarily dairy products) are also high in phosphorus content and must be restricted; calcium carbonate and calcium acetate may be used between meals to increase serum calcium levels. The K/DOQI Clinical Practice Guidelines for Bone Metabolism and Disease recommends 1.5 to 2.0 g of calcium (including dietary and supplemental calcium) for CKD stages 3 and 4 and 1.5 to 1.8 g for stages 4 and 5 not yet on dialysis. Goals are to keep serum calcium levels within normal range.
Table 10-6 Selected Phosphate-Binding Medications
Water Balance and Fluid Restriction
Fluid intake for individuals with CKD should be balanced by their ability to eliminate fluid. As long as urine output essentially equals the daily fluid intake, fluid balance is maintained. If edema becomes apparent, prescribing loop diuretics often increases sodium and water excretion sufficiently to maintain balance. In the latter stages of CKD, a fluid limit equal to the volume of urine output plus 500 mL/day for insensible fluid losses may be necessary to prevent edema and hyponatremia.
Vitamins and Iron
Protein and mineral restrictions to manage CKD usually result in a diet deficient in vitamins. Supplementation with folic acid (1 mg/day), pyridoxine (5 mg/day), the RDA for other B-complex vitamins, and ascorbic acid (60 to 100 mg/day) is often necessary. If the parathyroid hormone (PTH) level is above the goal range for the stage of CKD, a serum 25-hydroxyvitamin D level should be evaluated. If normal, it can be repeated on a quarterly basis; if less than 30∼ng/mL, supplementation with vitamin D2 (ergocalciferol) should be prescribed. Because of the kidney’s inadequate conversion of vitamin D from 25-hydroxycholecalciferol [25(OH)D] to its active form, 1,25-dihydroxycholecalciferol [1,25(OH2)D], supplementation of this active form or other analog of vitamin D is often required and individualized to keep serum PTH levels within goal range for the various stages of CKD. Vitamin A, on the other hand, may accumulate as CKD progresses and should not be supplemented. Vitamin preparations designed specifically for individuals with renal failure are available to meet patients’ needs.
Most patients with CKD develop anemia primarily because of the kidney’s decreased production of the hormone erythropoietin. This hormone stimulates the bone marrow to produce red blood cells. Many patients with CKD begin treatment with erythropoietin stimulating agents (ESAs) in the form of epoetin alfa or darbepoetin alfa prior to initiating dialysis. To promote red blood cell production, iron supplementation is often necessary for patients receiving erythropoietin therapy, but varies depending on iron status.
Dialysis
The goals of medical nutrition therapy for patients on maintenance HD, both in-center and home hemodialysis (HHD), and maintenance PD, both continuous ambulatory peritoneal dialysis (CAPD) and continuous cycling peritoneal dialysis (CCPD), are to maintain:
- protein equilibrium to prevent a negative nitrogen balance;
- serum potassium and sodium concentrations within an acceptable range and maintain total body sodium as close to normal as possible;
- fluid homeostasis by preventing fluid overload or volume depletion;
- serum calcium, phosphorus, and PTH levels within an acceptable range to prevent renal osteodystrophy and metastatic calcification; and
- adequate levels of vitamins and other minerals.
Medical Nutrition Therapy for Dialysis
Protein
Protein intake for patients undergoing maintenance dialysis must at least equal minimum dietary protein requirements but not worsen the uremic syndrome by causing retention of urea, electrolytes, and various minerals. The loss of amino acids, the catabolic stress of dialysis, and the level of protein intake in the pre-dialysis period may all contribute to poor protein status in the chronic dialysis patient. A protein allowance of 1.2 g/kg per day for in-center HD patients and 1.2 to 1.3 g/kg per day for HHD and PD patients will often minimize the accumulation of excessive nitrogenous wastes, maintain a positive nitrogen balance, and replace the amino acids lost during dialysis. During episodes of peritonitis, patients receiving PD have increased dietary protein needs due to greater losses of protein across an inflamed peritoneum. Many patients on both HD and PD periodically require supplemental commercial or homemade nutritional drinks, bars, or protein powders in order to achieve adequate protein intake.
Energy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree