16. Renal disease
Avril Collinson
LEARNING OBJECTIVES
By the end of this chapter the reader will be able to:
• Understand the delicate balance between the clinical chemistry and metabolic stability in renal disease;
• Assess the nutritional status of a renal patient and give appropriate intervention to optimise nutritional status; and
• Be familiar with the appropriate dietary advice for acute and chronic kidney disease.
Introduction
The optimal diet for renal disease has been the subject of much controversy in recent years. Low protein diets have been in and out of fashion for the treatment of chronic kidney disease (CKD). What is the latest advice? Markers of malnutrition are known to strongly predict morbidity and mortality rates in patients with renal failure; what nutrition interventions optimise nutritional status? Managing cardiovascular disease—what is the evidence for controlling lipid levels? The question of whether vitamin supplementation benefits renal patients is another area of much controversy, especially with the emerging importance of homocysteine.
This chapter will look at these complex and often controversial topics to piece together recommendations for the dietary management of CKD, using the latest available evidence. The five stages of CKD will be defined and the dietary recommendations for these stages will be discussed. The assessment of nutritional status in renal patients and the influence of diet on blood pressure will be reviewed. The dietary management of acute renal failure, nephrotic syndrome and renal transplantation will also be briefly outlined.
Physiology of the kidney
The kidneys, which are approximately 10 cm long and 5 cm wide, are located on the posterior wall of the abdomen at waist level. The kidney is a vital organ in the body and has a number of essential functions as shown in Table 16.1. The kidney allows a person to eat and drink as desired without causing disturbances in the composition of their intracellular and extracellular fluid compartments.
| Excretion of waste products (urea, creatinine, uric acid and other nitrogenous compounds) |
| Regulation of fluid balance |
| Regulation of blood pressure (renin–angiotensin mechanism) |
| Acid–base balance |
| Activation of vitamin D (25–hydroxycholecalciferol to 1,25-dihydroxycholecalciferol) |
| Production of erythropoietin (EPO) |
| Elimination and detoxification of drugs and toxins |
| Regulation of metabolic processes (gluconeogenesis, lipid metabolism) |
| Degradation and catabolism of hormones (insulin, glucagon, growth hormone, parathyroid hormone) |
| Electrolyte homeostasis (sodium, potassium, phosphate) |
| Regulating the metabolism of calcium and phosphorus to prevent renal osteodystrophy |
Classification of renal disease
Creatinine and urea are commonly measured as indicators of kidney function; however, both have their limitations. Creatinine is a metabolite from muscle and is excreted by the kidneys. An individual’s normal creatinine level will therefore depend on their muscle mass, age and gender. Urea is a small molecule produced in the liver from dietary protein consumed and is also excreted by the kidneys. As dehydration, dietary protein intake and liver disease can significantly influence urea levels, this test is an unreliable marker of renal disease.
Each kidney consists of approximately a million filters known as glomeruli. The glomerular filtration rate (GFR) is an estimate of the filtering capacity of the kidneys and is used as a more accurate measure of kidney disease. It is expressed as millilitres (mL) per minute (min) and adjusted to a ‘standard’ body size with a surface area of 1.73 m 2. The normal GFR range is between 90 and 130 mL/min/1.73m 2 but GFR is dependant on age, gender and body size. For example, if a GFR of 100 mL/min/1.73 m 2 is referred to as normal or 100% of kidney function, this means that a GFR of 30 mL/min/1.73m 2 represents approximately 30% of kidney function. From the age of mid-thirties a slow decrease of about 1% of GFR/year is generally seen. For example, at 70 years of age, the GFR is on average only 60–70% of the normal value.
In 1994, the Modification of Diet in Renal Disease Study Group (MDRD) published a study involving 1,628 patients that looked at the effects of dietary protein restriction and blood pressure control on kidney disease progression. 1 Using these results a formula that predicted GFR using demographic characteristics such as age, race, gender and the serum creatinine level was created. The MDRD formula was compared with the earlier Cockcroft-Gault prediction formula and found to correlate better with isotope GFR. 2 In addition it did not require the patient’s weight; hence, the MDRD formula is now the recommended method for estimating GFR (eGFR). 3
The US National Kidney Foundation-Kidney Disease Outcome Quality Initiative (NKF-K/DOQI) classification of CKD divides CKD into five stages based on GFR as shown in Table 16.2. 4 This classification, which enables the level of CKD in individual patients to be determined, has been adopted internationally. The National Institute for Health and Clinical Excellence (NICE) 2008 guidelines on CKD recommend Stage 3 CKD should be split into two subcategories as shown below and gives guidance on the management of CKD. 5 Progression of CKD is defined as a decline in eGRF of > 5 mL/min/1.73m 2 per year.
| aAlready known to have proteinuria, haematuria, microalbuminurea, polycystic kidney disease or reflux nephropathy, or a biopsy-proven chronic glomerulonephritis. | ||
| Stage | Description | GFR (mL/min/1.73 m 2) |
|---|---|---|
| 1 | Normal kidney function (GFR) but with another abnormalitya | > 90 |
| 2 | Mild impairment of kidney function but with another abnormalitya | 60–89 |
| 3A | Moderate impairment of kidney function | 45–59 |
| 3B | Moderate impairment of kidney function | 30–44 |
| 4 | Severe impairment of kidney function | 15–29 |
| 5 | Very severe or established renal failure (ERF) | < 15 |
The NHS is increasingly focusing on prevention, early detection and the treatment of renal disease. It is estimated that 30% of people with advanced kidney disease are referred late to nephrology services from both primary and secondary care and ultimately this is associated with significant cost and poor clinical outcomes. 5,6 The Quality and Outcomes Framework (QOF), a fundamental part of the new general practitioners contract, offers financial rewards for detecting CKD. As a result of the QOF, eGFR is now measured routinely in the community and more care at the earlier stages of CKD is being delivered by primary care. The NICE guidelines on CKD also promote the monitoring of kidney function in at-risk populations (individuals with hypertension, diabetes, vascular disease, urological abnormalities, family history of kidney disease or drugs affecting the kidney). 5
In Stage 5 where established renal failure (ERF) has been reached, many patients will require renal replacement therapy (RRT) to maintain life. RRT involves regular dialysis or a kidney transplant. ERF is an irreversible, long-term condition and some people with ERF will decide on conservative management only.
At the end of 2006, 43,901 adult patients were receiving RRT in the UK, a population prevalence of 725 pmp, an increase from 694 pmp in 2005. Of these patients receiving RRT, 45% had a transplant, 43% were on centre-based haemodialysis (HD), 1% on home haemodialysis and 11% on peritoneal dialysis (PD). The median age of patients starting RRT was 65 years. The data reported here have been supplied by the UK Renal Registry of the Renal Association. 7
As well as being a marker of the progression of CKD, eGFR is thought to be a powerful predictor of cardiovascular risk. 8 Estimated GFR should be interpreted with caution in patients where there are extremes of muscle mass, for example, in severe cases of muscle wasting or bodybuilders. In these patients a reduced muscle mass will lead to an overestimation and an increased muscle mass will lead to an underestimation of the eGFR. Ethnicity should also be considered. For example, people of Afro-Caribbean origin tend to have a greater muscle mass as compared to non Afro-Caribbeans and so it is recommended that eGRF is multiplied by 1.21 to correct for this.
Causes and incidence of renal disease
CKD is now recognised as a global public health problem. 9 Diabetes and hypertension are well-known risk factors for renal disease. Body mass index has been found to be an independent predictor for CKD after adjustments for blood pressure level and presence or absence of diabetes mellitus. 10 Two studies in the UK have reported a higher incidence of CKD in areas with higher social deprivation scores. 11
Current estimates suggest that 5–11% of the global population have CKD, defined as a GFR < 60 mL/min/1.73 m 2. 12,13 A figure of 8.5% has been quoted for the prevalence of stage 3–5 CKD in the UK. 14 In the UK estimates of CKD stage 3 are 4.6–4.8%; however, prevalence is associated with age, as 30.5% of men and 37.5% of women in their 80s are estimated to have CKD stage 3. 15 The question is asked as to whether a low GFR in elderly people is just a normal part of the physiological process of ageing and some question the appropriateness of applying GFR to all ages. The prevalence of CKD has been reported as higher in certain ethnic minority groups in the UK.
Table 16.3 shows the main causes of ERF as taken from the UK renal registry data 2006. 7 Worldwide these figures are known to vary considerably.
| aBiopsy proven. | |
| Diabetes | 22.2% |
| Glomerulonephritisa | 10.4% |
| Pyelonephritis | 7.2% |
| Renal vascular disease | 6.8% |
| Polycystic kidney | 6.7% |
| Hypertension | 5.4% |
Slowing the progression of renal disease
There are many ways to slow the progression of renal disease.
Hypertension
Hypertension is a cause of CKD, but it is also a consequence of failing kidneys so the control of blood pressure is important. The use of angiotensin converting enzyme (ACE) inhibitors and/or angiotensin receptor blockers (ARBs) is considered to be the most effective in slowing the progression of CKD. 16 Proteinuria as a result of increased leakage from the glomeruli or from a decreased tubular reabsorption is a marker of CKD, and may cause progression of CKD. 17 Albumin:creatinine ratio (ACR) is the test of choice to identify proteinuria in people with diabetes and is already widely used in practice. Albumin is the predominant component of proteinuria in glomerular disease and the ACR test is considered to be a sensitive test for detecting early CKD.
NICE guidelines for recommended blood pressure (BP) targets for CKD are < 140/90 and < 130/80 mmHg in patients with diabetes or those with an ACR > 70 mg/mmol or urinary protein excretion > 1 g/24 h. 5
Dietary management of hypertension
A consistent body of evidence from observational studies and clinical trials indicates that body weight is positively associated with BP and hypertension. A meta-analysis of randomised controlled trials (RCTs) revealed that a weight loss of approximately 5 kg led to a reduction of about 4.4 and 3.6 mmHg in systolic and diastolic BP, respectively. 18 Expressed per kilogram of weight loss this equates to a reduction of 1.05 mmHg in systolic and 0.92 mmHg in diastolic. Another high-quality systematic review in hypertensive individuals suggested that dietary measures which achieve a 3–9% reduction in body weight are likely to reduce systolic and diastolic pressure by about 3 mmHg. 19
The relationship between salt and BP is often difficult to establish as accurate intakes of sodium intake or excretion are hard to obtain, and adherence to a salt restriction can often be poor. Some researchers believe individuals should be classified as salt-sensitive or resistant. 20 Although at present there is no way of detecting which individuals are salt sensitive, salt sensitivity appears to be more frequent amongst individuals with hypertension and/or diabetes, in black and/or older individuals. 21,22 Some believe that salt sensitivity is a transient phenomenon during the pathogenesis and development of hypertension. 23 Since the phenomenon of salt sensitivity has not been characterised it is not yet possible to identify and develop predictive markers, including genetic polymorphisms, for individuals.
This phenomenon may explain some of the variations found in the literature; however, many findings from epidemiological and intervention studies do conclude a positive relationship between salt and BP. 24.25. and 26. There is also a suggestion by some researchers that salt exposure is linked with kidney tissue injury. 27
Several epidemiologic studies have established that dietary potassium intake is inversely related to BP. 28 An increase in potassium consumption has been shown to lower BP or reduce antihypertensive medication, especially in people who are hypertensive. 29,30 Dietary recommendations should therefore advise on an increase in fruit and vegetable consumption to help reduce BP, unless blood potassium levels are raised. This can often lead to confusion in CKD patients as they may be advised later on to reduce their intake of fruit and vegetables to help control their blood potassium levels.
For further information on hypertension please refer to the chapter on cardiovascular disease.
Diabetes
In diabetic patients, keeping glycated haemoglobin (HbA1c) levels to normal or near normal (<7.5%) has been shown to either delay the onset of diabetic nephropathy or slow the progression. 31.32. and 33. For further information on glycaemic control please refer to the chapter on diabetes.
Cardiovascular disease
The great majority of patients with early CKD will have an increased risk of cardiovascular disease (CVD). 34 It has been stated that patients with a GFR < 60 mL/min/1.73 m 2 have a higher risk of cardiovascular death, and 74% of patients on dialysis have heart damage. 35.36. and 37. The risk of CVD in patients with CKD far outweighs the risk of progression of the disease. A retrospective cohort study found that only 4% of 1,076 individuals progressed to ERF over a 5.5-year follow-up period, 69% had died at the end of follow-up and the cause of death was cardiovascular in 46% of cases. 11
In the general population the relationship between dyslipidaemia and CVD is well known; however, in CKD patients it is still controversial. Although dyslipidemia has been associated with CKD, it is still uncertain whether lipid-lowering therapy will reduce CVD. The spectrum of dyslipidaemia in CKD is distinct from the general population and the optimal targets for plasma lipids in people with CKD are not yet known. Lipid levels are found to vary with the stage of CKD and presence of diabetes and/or nephrotic syndrome. 38.39.40. and 41. Lipoprotein(a) is also influenced by CKD and the degree of proteinuria. 38,42
Lipid-lowering therapy has been found to modestly reduce the rate of kidney function loss in patients with moderately impaired kidney function (stage 3A) with or at risk of CVD. 43 A number of other researchers have also suggested that managing risk factors for CVD may slow the rate of decline of kidney function. 44,45 Evidence for the beneficial effects of lipid therapy on reducing CVD risk in CKD is, however, scarce. A randomised, placebo-controlled, intervention trial on lipid-lowering therapy in ERF patients with diabetes showed no significant reduction in primary endpoint of cardiovascular death, nonfatal myocardial infarction or stroke by statin therapy. 46 One explanation for this could be a higher significance of structural heart disease (e.g. vascular calcification, left ventricular failure) rather than classical myocardial infarction in advanced kidney disease. Results of a larger clinical trial, the Study of Heart and Renal Protection (SHARP), which is currently investigating 9,000 CKD patients will hopefully help to answer the question of whether lipid-lowering therapy is beneficial in CKD. 47
The NICE guidelines on CKD currently recommend that people with CKD and coronary disease should be treated according to existing guidelines and those who do not have evidence of coronary disease should be treated according to their estimated risk, using the Joint British Societies Guidelines. 5 Statins should be offered for the secondary prevention of CVD irrespective of baseline values.
Anaemia
Anaemia is very common in CKD due to a reduction in erythropoietin production. There is increasing evidence that correcting anaemia may have favourable effects on the progression of CKD. 48 Guidelines for haemoglobin targets have been developed, but there is no consistent agreement on the appropriate level . NICE recommend anaemia treatment should aim to maintain stable haemoglobin levels between 10.5 and 12.5 g/dL. One study, the Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR), investigating whether achieving higher haemoglobin targets of 13.5 g/dL is beneficial was terminated as findings suggested an increased risk of CVD outcomes with these higher levels. 49
Protein restriction
In the 1980s a number of animal studies showed that a low protein diet improved survival and slowed the progression of renal failure. 50.51. and 52. A meta-analysis in 1992 concluded that the use of low protein diets in humans also slowed the progression of renal disease. 53 In this meta-analysis 46 trials were looked at but only 6 reports of RCTs were selected. Many of the earlier human studies reporting benefits of low protein diets were flawed in their methodological approach, compliance with the prescribed protein intake was poor and little or no nutritional assessment was undertaken.
Results of two large multicentred studies were published in the 1990s. The Northern Italian Cooperative Study investigated 456 patients and found no difference in creatinine clearance when patients were randomly allocated protein intakes of either 1 g/kg or 0.6 g/kg body weight for a 2-year period. In this study the authors concluded that the underlying disease was more influential than the dietary changes but noted that compliance was poor with the 0.6 g/kg protein diet. 54
The other study published was the MDRD study from the USA. 1 This trial operated as two concurrent studies, involving a total of 840 patients. In study 1 patients with a GFR of 25–55 mL/min/1.73 m 2 were randomly assigned to a usual protein diet (1.3 g/kg/day) or a low-protein diet (0.58 g/kg/day). Patients in study 2, with a GFR of 13–24 mL/min/1.73 m 2, were randomly assigned to the low protein diet or to a very low protein diet (0.28 g/kg/day) with a keto acid–amino acid supplement. No significant difference was found between the groups in the rate of decline of renal function in study 1. In study 2 the very low protein group had a marginally slower decline in glomerular filtration than the low protein group but there was no difference in the time to reach ERF or death.
As there were problems with compliance with the prescribed protein intake in the MDRD study, a secondary analysis was undertaken to examine the relationship between actual achieved protein intake and the rate of decline of renal function. 55 This secondary analysis pointed to a correlation between actual protein intake and a decreased rate of decline in GFR. However, as the analyses were made by correlation rather than ‘intention to treat’, this finding cannot be directly applied to the clinical setting.
A Cochrane review published in 2007 looked at 12 studies, including 9 RCTs, to assess the effects of protein restriction in diabetic renal patients. 56 The authors concluded that reducing protein intake appears to slightly slow progression of diabetic kidney disease but this is not statistically significant. The authors commented that individual variation existed so protein restriction may be beneficial in some individuals. No data were found on the effects of low protein diets on health-related quality of life.
Despite much research, the area of protein restriction and its possible effect of slowing the progression of CKD still remain controversial. Suggested reasons for limiting the protein intake of CKD patients include reducing uraemic symptoms, reducing acidosis and proteinuria and slowing the decline of kidney damage. Limitations over restricting protein intake have arisen, firstly, as concerns over a deterioration in nutritional status and associations between hypoalbuminaemia and increased mortality, and secondly because many patients already self-restrict their protein intake through imposed dietary restrictions or due to a loss of appetite and generally feeling unwell. 57,58 In view of the conflicting evidence, nutritional status and quality of life issues many UK units currently choose a moderate protein restriction of 0.8–1 g protein/kg ideal body weight.
Dietary advice for CKD
A number of guidelines have been published for the nutritional management of CKD.
• National Kidney Foundation–Kidney Disease Outcome Quality Initiative (NKF-K/DOQI), Clinical Practice Guidelines for Nutrition of Chronic Renal Failure (USA 2000). 59
• American Dietetic Association (ADA) Medical Nutrition Therapy Evidence-Based Guides for Practice, Chronic Kidney Disease (Non-dialysis) Medical Nutrition Therapy Protocol (2002). 60
• ADA Guidelines for Nutritional Care of Renal Patients, 3rd edn (2002). 61
• European Dialysis and Transplantation Nurses Association/European Renal Care Association (EDTNA/ERCA), Guidelines for the Nutritional Care of Adult Renal Patients (2003). 62
• Caring for Australians with Renal Impairment (CARI), Nutrition and Growth in Kidney Disease Guidelines (2005). 63
• European Society for Clinical Nutrition and Metabolism (ESPEN), Guidelines on Enteral Nutrition: Adult Renal Failure (2006). 64
• Evidence Based Practice Guidelines for the Nutritional Management of CKD (Australia/New Zealand 2006). 65
The aims of dietary treatment for CKD are:
• To limit the build-up of waste products and help maintain fluid/electrolyte balance (urea, phosphate, potassium, fluid and salt);
• To prevent metabolic complications, e.g. renal bone disease, acidosis, anorexia, obesity;
• To attempt to delay the progression of renal failure;
• To optimise/maintain nutritional status; and
• If on dialysis, to replace nutrient losses associated with the dialysis process (nitrogen, vitamins and minerals).
Table 16.4 summarises an adapted version of the European guidelines for the nutritional care of adult renal patients. It is important to ensure that any nutritional restrictions do not compromise nutritional adequacy and quality of life. There is no agreed consensus for estimating ideal body weight (IBW) in patients outside of the normal BMI range and clinical judgement is often used. The European guidelines recommend that for a patient who is overweight (BMI > 25) IBW is calculated as the weight at a BMI of 25. Conversely if a patient is underweight (BMI < 20) IBW is calculated as the weight at a BMI of 20.
| aIBW = Ideal body weight. | |||
| bPDUO = volume equal to previous day’s (24 hr) urine output. | |||
| Adapted from the European Dialysis and Transplantation Nurses Association/European Renal Care Association (Dietitians Special Interest Group guidelines). 62 | |||
| Pre-dialysis CKD stage 3–4 | Haemodialysis CKD stage 5 | Peritoneal dialysis CKD stage 5 | |
|---|---|---|---|
| Energy kcal | 35 kcal/kg IBWa (30–35 kcal/kg IBW depending on age and activity) | 35 kcal/kg IBW (30–35 kcal/kg IBW depending on age and activity) | 35 kcal/kg IBW including calories from peritoneal absorption of glucose (30–35 kcal/kg IBW depending on age and activity) |
| Protein | 0.6–1 g/kg IBW If < 0.8 ensure sufficient dietetic follow-up and > 55% HBV | 1–1.2 g/kg IBW | 1–1.2 g/kg IBW 1.5 g/kg IBW (peritonitis) |
| Phosphorus | 600–1000 mg/day (19–32 mmol/day) | 1000–1400 mg/day (32–45 mmol/day) | 1000–1400 mg/day (32–45 mmol/day) |
| Potassium | 2000–2500 mg/day (50–65 mmol/day) | 2000–2500 mg/day (50–65 mmol/day) | If restricted 2000–2500 mg/day (50–65 mmol/day) |
| Sodium | 1800–2500 mg/day (80–110 mmol/day) | 1800–2500 mg/day (80–110 mmol/day) | 1800–2500 mg/day (80–110 mmol/day) |
| Fluid | Restrict if required | 500 mL plus PDUOb | 800 mL plus PDUO |
Variations do exist between the different guidelines; for example, the USA K/DOQI guidelines suggest a protein intake of 0.6–0.75 g/kg (at least 50% high biological value, HBV) for CKD stage 1–4 as compared to the Australia/New Zealand recommendation of 0.75–1 g protein (> 50% HBV) for CKD stage 3–4. For the calculation of IBW the Australia/New Zealand guidelines recommend that a modified BMI range of 23–26 should be taken as the normal range if a patient is on dialysis. 65
CKD stages 1–4
Protein and energy
The incidence of protein energy malnutrition (PEM) increases with deteriorating kidney function and is associated with a poor outcome; therefore early dietetic intervention is important. 58 Most patients with a GFR < 30 should be referred to a dietitian. In view of the conflicting evidence around protein restriction many UK units choose a moderate protein restriction of 0.8–1 g protein/kg IBW. Renal dietitians will use protein exchanges to assess a patient’s intake. Either 6 or 7 g protein exchanges are used for HBV protein and 2-g exchanges for low biological value (LBV) protein. It may not be appropriate to teach these to the patient. As patients become uraemic and symptomatic they often self-restrict protein anyway. Symptoms of uraemia can include loss of appetite, nausea and vomiting, taste changes, tiredness and itching.
An energy intake of 35 kcal/kg IBW is recommended to prevent nitrogen catabolism; however, obesity should be avoided as a BMI > 30 is associated with a variety of health problems. There are no randomised trials examining the safety and efficacy of low-fat diets in patients with CKD although dyslipidaemia is evident in a number of patients. Where possible lipid-lowering dietary measures should be advised when this is not at the detriment of the patient’s nutritional status. Tight glycaemic control is essential in patients with diabetes to help slow the progression of CKD.
Potassium
The risk of developing hyperkalaemia is inversely related to renal function and it is only when at least 50% of the kidney function has been lost that the blood biochemistry is affected. Hyperkalaemia may occur as a result of impaired tubular secretion of potassium in patients with mild CKD. It is important for the dietitian to be aware that a number of other factors can contribute to hyperkalaemia: catabolism, certain medications (i.e. ACE inhibitors, potassium sparing diuretics, angiotensin receptor blockers), metabolic acidosis, blood transfusions, dehydration and constipation. Metabolic acidosis can develop with the progression of renal disease, and results in both hyperkalaemia and increased protein catabolism. Oral bicarbonate supplementation (or increasing the dialysate bicarbonate concentration if on dialysis) may be given to correct this, although this may contribute to greater sodium retention and hypertension.
Patients with serum potassium levels over 5.5 mmol/L are usually targeted for dietary advice if hyperkalaemia cannot be corrected by other means. It is important to check local guidelines for normal potassium ranges. Generally, a slow rise in serum potassium level, as seen with CKD, is tolerated better than an abrupt rise in potassium levels. Many patients with hyperkalaemia are asymptomatic, but the consequences of hyperkalaemia can be fatal as listed below:
• Paraesthesia in the extremities;
• Muscle weakness leading to possible flaccid paralysis;
• Diarrhoea due to smooth muscle hyperactivity; and
• Cardiac arrythmias and cardiac arrest.
Dietary advice for potassium restriction should be individually tailored to the patient. Advice should focus on altered cooking methods, suitable portion sizes and lower potassium food sources. As potassium is water-soluble one example of an altered cooking method is to recommend boiling potatoes and vegetables in large amounts of water instead of cooking in the microwave or steaming. The vegetable water must not be used to make gravy, soups or sauces.
If the serum potassium is slightly raised, only a few dietary changes may be advised although the patient should still be educated on which foods are high in potassium to avoid further problems. Table 16.5 gives a list of foods high in potassium which are normally limited and examples of lower potassium alternatives. The use of 4-mmol potassium exchanges can be useful for the renal dietitian and some patients may also benefit from this knowledge to allow more variety in their diet. Other patients may find this method too complex.
| aBoiled potatoes usually limited to 125 g (5 oz) once/day. | |
| bMilk usually limited to ½ pint/day. | |
| High potassium foods | Lower potassium alternatives |
|---|---|
| Fruit juice | Squash drinks |
| Banana/grapes | Apple |
| Dried fruit | |
| Potatoes especially chips, sauté and jacket potato | Rice, pasta, boiled potatoesa |
| Potato crisps | Corn chips |
| Mushrooms | Cauliflower |
| Tomatoes | Carrots |
| Nuts | Popcorn |
| Chocolate | Sweets/mints |
| Fruit cake | Doughnut/sponge cake |
| Beer/wine | Spirits |
| Coffee | Tea |
| Milkb | |
| Soups | |
| Potassium-containing salt substitutes | |
Potassium restrictions should only be advised if absolutely necessary as once patients have been advised to avoid certain foods they will often find it difficult to reintroduce these foods again. Remember to check the biochemistry first to see whether potassium restrictions are really required.
Phosphorus and renal bone disease
The kidneys play a crucial role in regulating serum phosphate and calcium homeostasis, both directly and through the activation of vitamin D. Problems start to occur with the control mechanisms essential for calcium and phosphate homeostasis early in the course of CKD, before any abnormalities are seen in the biochemistry, and continue to progress as kidney function decreases. 66 As CKD advances, phosphate retention occurs and hypocalcaemia develops as the kidneys fail to activate vitamin D. Both hypocalcaemia and hyperphosphataemia stimulate an increase in the parathyroid hormone (PTH). 67,68 This condition shown in Figure 16.1 is known as secondary hyperparathyroidism. The excess parathyroid hormone then acts on bone to increase the release of calcium and phosphate. The blood levels of calcium and phosphate then increase, while the bones become weaker, a condition known as renal bone disease. Excess phosphate binds with calcium to form calcium phosphate which can be deposited in the heart, lungs, kidneys and other soft tissues. This is referred to as soft tissue calcification. There is evidence that poor calcium and phosphate control affect morbidity and mortality due to cardiovascular calcification. 69 Although many patients complain of itching, most are not aware of these serious developments until they experience bone pain and fractures.
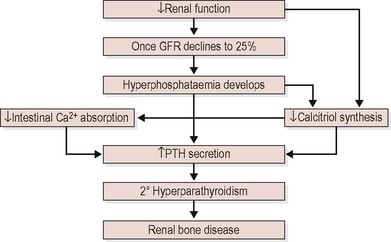 |
| Figure 16.1 |
Treatment for suppressing PTH levels includes a dietary phosphorus restriction, phosphate binders to limit the absorption of phosphorus and supplementation with active vitamin D and calcium-containing phosphate binders to help correct hypocalcaemia. In practice dietary phosphorus is normally referred to as phosphate to avoid confusion for patients.
Dietary phosphorus is mainly found in protein-rich foods as shown in Box 16.1. When advising on restrictions care should be taken that patients are not put at risk of PEM. Providing that the patient is not malnourished it is believed to be beneficial to consider a dietary phosphorus restriction early. PTH levels begin to rise when GFR falls below 60 mL/min/1.73 m 2 (CKD stage 3), even though serum phosphorus levels are normal, so levels of PTH are believed to be a better indicator of when to begin a dietary phosphorus restriction. K/DOQI clinical practice guidelines on bone metabolism and disease in CKD recommend that dietary phosphorus restrictions should be initiated when blood PTH levels begin to rise and/or when serum phosphate levels are elevated at any stage of CKD (normally > 1.4 mmol/L). 70
 Box 16.1
Box 16.1 Cheese
Milk
Yoghurt
Poultry
Meat, particularly offal
Fish, particularly oily fish
Seafood
Eggs
Nuts
Chocolate
Often a dietary phosphorus restriction is not sufficient to control phosphate levels so phosphate binders are required. Phosphate binders bind with phosphate in the digestive tract to form a compound that is not absorbed. It is therefore important that they are taken with meals and snacks which provide dietary phosphorus. There are three common types of phosphate binders: aluminium-based, calcium-based and aluminium-free/calcium-free phosphate binders.
Aluminium-based phosphate binders are very effective at controlling phosphorus. The most common binder of this type is aluminum hydroxide; however, aluminium is known to have toxic effects that can cause bone disease and damage the nervous system, so for this reason, aluminium-based phosphate binders are rarely used.
Calcium-based phosphate binders can be effective but do not bind phosphorus as well as aluminium. Common types of calcium-based binders used are calcium acetate and calcium carbonate. These binders can also serve as calcium supplements but taking too many will lead to an excess calcium load which can contribute to metastatic calcification in tissues and organs. Cardiac calcification can lead to heart damage and even death.
Aluminium-free/calcium-free phosphate binders, for example, sevelamar and lanthanum carbonate, are newer, more costly binders which have recently been introduced.
The appropriate prescription including the selection, dose and timing of phosphate binders is important for good phosphate control. The dietitian is the team member best suited to recommend when and how many phosphate binders patients should take. In some hospitals treatment protocols and patient group directions which allow the dietitian to both initiate and adjust phosphate binders independently of medical staff have been developed. These changes have been associated with improvements in patient care resulting in significantly better bone biochemistry. 71
Sodium and fluid homeostasis
As kidney function deteriorates less sodium is excreted. The K/DOQI Clinical Practice Guidelines on Hypertension and Antihypertensive Agents in Chronic Kidney Disease recommend that most CKD patients should reduce sodium intake to 100 mmol/day to reduce extracellular fluid volume expansion and lower BP. 72 This is similar to the EDTNA/ERCA guidelines of 80–110 mmol/day, which is equivalent to a ‘no added salt’ diet. 62 A ‘no added salt’ diet is achieved by avoiding or minimising salt in cooking, not adding salt at the table and avoidance of too many processed foods. Salty foods, e.g. smoked or cured foods, should also be limited as much as possible. The use of herbs and spices are encouraged for flavouring. Patients are advised to avoid potassium-containing salt substitutes. Rarely some patients can be salt losers, identified by urinary sodium excretion, and may need sodium supplementation.
The recommended fluid intake for the majority of CKD patient’s stage 1–4 is no different from the general population (30–35 mL/kg/IBW) as urine output is not reduced. However, a mild to moderate fluid restriction may be required for some patients especially if resistant oedema is present.
ERF (CKD stage 5)
Dialysis
Haemodialysis and peritoneal dialysis are only equivalent to approximately 10–15% of normal kidney function.
Peritoneal dialysis (PD)
In peritoneal dialysis (Figure 16.2) a Tenckhoff catheter is inserted into the peritoneal cavity and a ‘glucose-based’ dialysate is drained into the peritoneal cavity. The peritoneal membrane surrounding the intestine acts as a natural semi-permeable membrane. Uraemic toxins are removed from the blood into the dialysate by diffusion and fluid is removed by osmosis. In continuous ambulatory peritoneal dialysis (CAPD) the dialysate is drained into the peritoneum and after 4–6 hours drained out again. This process or exchange is repeated 4 or 5 times a day. Volumes of dialysate are 1–3 L and vary in concentration. Table 16.6 shows examples of different strengths of dialysate bags and the estimated energy absorbed from these bags. To optimise diabetic control and reduce peritoneal membrane exposure to glucose dialysis prescription regimens that incorporate less glucose and more glucose-free (amino acid, icodextrin) solutions are recommended.
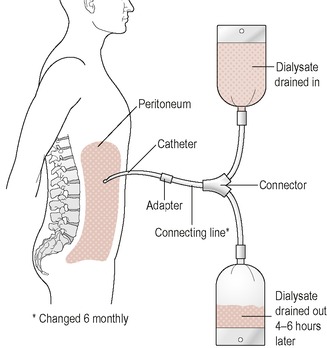 |
| Figure 16.2 |
| Strength of 2-L bag | Approximate energy absorbed |
|---|---|
| 1.36% dextrose (light) | 70 kcal |
| 2.27% dextrose (medium) | 120 kcal |
| 3.86% dextrose (heavy) | 200 kcal |
In continuous cycler-assisted peritoneal dialysis (CCPD), also referred to as automated peritoneal dialysis (APD), a machine is programmed to carry out the exchanges usually overnight. The machine controls the fill volume, dwell time and length of treatment. This allows larger volumes of dialysate to be processed (up to 20 L). The patient usually has 2 L of dialysate fluid left in during the day and may need to carry out additional daytime manual exchanges.




