and Paul Telfer2
(1)
Department of Haematology, Guy’s and St Thomas’ Hospital, London, UK
(2)
Department of Haematology, Royal London Hospital, London, UK
Renal Dysfunction/Proteinuria
Pathophysiology
The kidney is susceptible to damage through several mechanisms which may work independently or in tandem. The disease process may effect glomerular and proximal tubular function as well as the urinary concentrating mechanism in the medulla. HbSS patients are generally the most severely affected, but significant pathology is also frequently observed in adults with HbSC, and even in carriers of SCD (See Chap. 1). A distal renal tubule concentrating defect is usually the first manifestation of renal damage, resulting in hyposthenuria (an inability to concentrate urine). Increased renal cortical blood flow due to anemia and high cardiac output is apparent from early childhood, and is associated with an increased glomerular filtration rate (GFR). Cortical and medullary ischaemia may also contribute to the increase in GFR. This glomerular hyperfiltration causes glomerular hypertrophy and is partly responsible for the progressive glomerular damage, which manifests initially as microalbuminuria and at later stages, proteinuria and progressive renal impairment. Histologically the glomeruli become enlarged and congested with sickled red cells, and there is proliferation of the mesangium (Fig. 8.1). Later on the changes of focal and segmental glomerulosclerosis (FSGS) may develop.
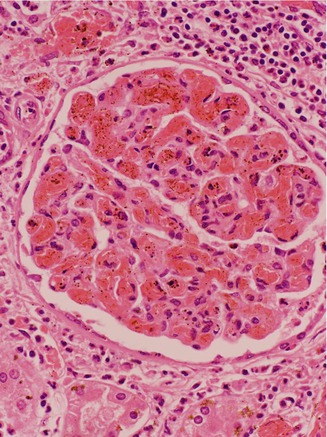

Figure 8.1
Renal biopsy showing an abnormal glomerulus which is enlarged with congested vessels and thickened mesangium
In the medulla, there is occlusion of the vasa recta, (the vessels involved in the counter-current urinary concentration mechanism), together with ischemic damage and fibrosis. These changes are probably due to enhanced sickling in medullary conditions of slow blood flow, hemoconcentration, acidosis and low oxygenation.
In adults over the age of 30 there tends to be a progressive drop in GFR, and the insidious development of renal failure.
Prevalence
Microalbuminuria is present in up to 21 % of children and over 60 % of adults. The prevalence of proteinuria is around 4 % of children, 10 % of teenagers, 20 % of young adults and over 40 % in adults over 40 years of age. Renal insufficiency is seen in over 21 % of adults and progresses to end stage renal failure in 4-12 % of patients. It is likely that as survival and life expectancy improve, renal disease will become more common.
Clinical Presentation
Children who develop a concentrating defect pass large volumes of urine and often have problems with nocturnal enuresis, persisting into the teenage years. Adults may have nocturia, which can be associated with increasing proteinuria. Progressive loss of renal function occurs without noticeable symptoms and patients may present to clinic with end-stage renal failure.
Diagnosis and Screening
Screening and risk management for renal disease should be part of the routine care for children and adults and we recommend that these are part of the annual review. The assessment should include blood pressure monitoring, measurement of creatinine and eGFR and quantification of proteinuria by albumin-creatinine or protein-creatinine ratio (See Chap. 19). Children or their carers should be questioned about enuresis, hematuria and urinary infections.
Creatinine levels are usually low in SCD and a trend of increase over time, even if still within the normal range, may indicate worsening renal function. Patients presenting with proteinuria or chronic renal dysfunction should have investigations to rule out alternative causes including autoimmune disorders, chronic viral infection and structural abnormalities in the renal tract (demonstrable by ultrasound).
Management
These patients should be reviewed by a renal physician with an interest in SCD, preferably in a specialist sickle-renal clinic. Renal biopsy should be considered, particularly in those with an atypical presentation or features which may suggest an alternative diagnosis. Recommendations for treatment are shown in Table 8.1.
Table 8.1
Recommendations for screening and treatment of renal disease
Test result | Treatment option |
|---|---|
Microalbuminuria (ACR >2.5 mg/mmol men, >3.5 mg/mmol women or PCR >15 mg/mmol) | No treatment but monitor regularly for progression |
Proteinuria (ACR >30 mg/mmol or PCR >50 mg/mmol) which is persistent | Investigate for other causes of renal dysfunction. Commence ACEi or ARB and dose escalate if required |
Hypertension >140/90 mmHg OR >130/80 mmHg if patient proteinuric | Treat with calcium antagonist or beta blocker as first line agent aiming for blood pressure of <130/80 mmHg (or <120/70 mmHg if proteinuric). ACEi may be a suitable alternative |
Progressive anemia with renal impairment (Hb <65 g/l, reticulocytes <150 x109/l, eGFR <60 ml/min) | Trial of ESA. If ineffective after dose escalation, regular top-up transfusion may be required. Hemoglobin should not exceed 100 g/l |
Angiotensin-Converting Enzyme Inhibitors (ACEi)
These drugs act by inhibiting the Angiotensin II- mediated constriction of efferent glomerular arterioles, thereby decreasing the intra-glomerular pressure and glomerular permeability to albumin. The result is a decrease in proteinuria and normalization of glomerular filtration rate. This specific anti-proteinuric effect is combined with the action in controlling blood pressure. In diabetes mellitus, both ACEi and angiotensin receptor blockers (ARBs) have shown a similar anti-proteinuric effect and they have also been shown to slow the progression of renal impairment. There is some evidence that they also decrease proteinuria and microalbuminuria in patients with HbSS, and may also slow renal impairment. RCTs currently underway will help to define the role of these agents in managing renal impairment in SCD.
Hydroxyurea
The role of hydroxyurea in protecting against renal damage is not known. One of the primary endpoints of the BABYHUG trial was a reduction in GFR as measured by radionucleotide renal clearance. After 2 years of follow-up, GFR was increased to the same level in hydroxyurea and placebo groups. Although this result does not support hydroxyurea for routine use in preventing renal damage in early childhood, it does not rule out a potential role in longer-term control of renal disease in children and adults, and further controlled trials will be important to investigate this. Smaller case series suggest that hydroxyurea may be beneficial in selected patients. If it is to be used in those with established renal impairment, dose reduction is needed to avoid toxicity.
Blood Pressure Control
Blood pressure control is essential for patients with evidence of renal dysfunction. A target value of <130/80 is reasonable in adult patients who are proteinuric.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree