Chapter 11 Regulation of the Immune Response
• Many factors govern the outcome of any immune response. These include the antigen itself, its dose and route of administration, and the genetic background of the individual responding to antigenic challenge. A variety of control mechanisms serve to restore the immune system to a resting state when the response to a given antigen is no longer required.
• The APC has an important effect on the immune response through its ability to provide co-stimulation to T cells and by the production of cytokines and chemokines that influence both the nature and make-up of the ensuing reponse. In addition, APC heterogeneity aids in the promotion of different modes of immune response.
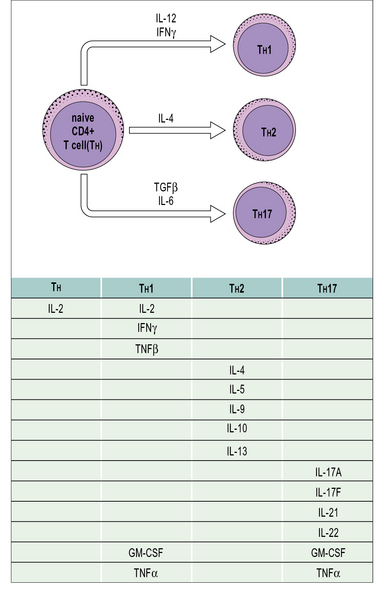
• T cells regulate the immune response. Cytokine production by T cells influences the type of immune response elicited by antigen. CD4+ T cells can differentiate into several effector phenotypes such as TH1, TH2 or TH17. These subsets play important roles in the protection of the host against a diversity of pathogen challenges. Regulatory T cells may belong to the CD4 or CD8 subpopulations. They may inhibit responses via a variety of mechanisms such as via cell-to-cell contact or by the production of the anti-inflammatory cytokines IL-10 and TGFβ.
• Immunoglobulins can influence the immune response. They may act positively, through the formation of immune complexes, or negatively, by reducing antigenic challenge or by feedback inhibition of B cells.
• Selective migration of lymphocyte subsets to different sites can modulate the local type of immune response because different TH subsets respond to different sets of chemokines.
• The neuroendocrine system influences immune responses. Cells from both systems share similar ligands and receptors, which permit cross-interactions between them. Corticosteroids in particular downregulate TH1 responses and macrophage activation.
• Genetic factors influence the immune system and include both MHC-linked and non-MHC-linked genes. They affect the level of immune response and susceptibility to infection.
Ideally, an immune response is mounted quickly to clear away a pathogenic challenge with the minimum of collateral damage and then the system is returned to a resting state once the antigen is eliminated. The immune response, like many other biological systems, is therefore subject to a variety of control mechanisms. Additional mechanisms help regulate the levels of immunopathology that are often a necessary sacrifice for pathogen elimination. An insufficient immune response can result in an individual being overwhelmed by infection. An inappropriate, or over vigorous immune response can lead to high levels of immunopathology or even autoimmunity (see Chapter 20). The balance between these two is therefore critical.
• the form, dose, and route of administration of the antigen;
• the antigen-presenting cell (APC);
• the genetic background of the individual;
• any history of previous exposure to the cognate antigen; and
Specific antibodies may also modulate the immune response to an antigen.
Regulation by antigen
Different antigens elicit different kinds of immune response
In some situations, antigens (e.g. those of intracellular microorganisms) may not be cleared effectively leading to a sustained immune response. Chronic immune responses have several possible pathological consequences and can lead to autoimmunity and hypersensitivity (see Chapters 20 and 23–26).
Large doses of antigen can induce tolerance
Very large doses of antigen often result in specific T – and sometimes B – cell tolerance.
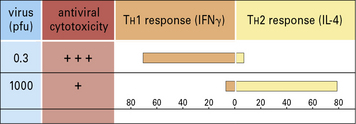
Administration of antigen to neonatal mice often results in tolerance to the antigen. It was once speculated that this might be the result of immaturity of the immune system. However, neonatal mice can develop efficient immune responses (Fig. 11.1) and their non-responsiveness may in some cases be attributable, not to the immaturity of T cells, but to immune deviation. In this case a non-protective TH2-type cytokine response would dominate a protective TH1-type cytokine response.
T-independent polysaccharide antigens have been shown to generate tolerance in B cells after administration in high doses. Tolerance and its underlying mechanisms are discussed in Chapter 19.
Antigen route of administration can determine whether an immune response occurs
The route of administration of antigen has been shown to influence the immune response:
• antigens administered subcutaneously or intradermally evoke an active immune response; whereas
• antigens given intravenously, orally, or as an aerosol may cause tolerance or an immune deviation from one type of CD4+ T cell response to another.
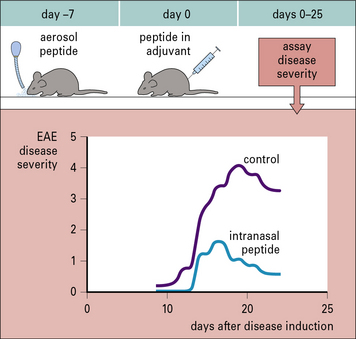
Similar observations have been made when antigen is given as an aerosol. Studies in mice have shown that aerosol administration of an encephalitogenic peptide of myelin basic protein (MBP) inhibits the development of experimental allergic encephalomyelitis (EAE) that would normally be induced by a conventional (subcutaneous) administration of the peptide (Fig. 11.2).
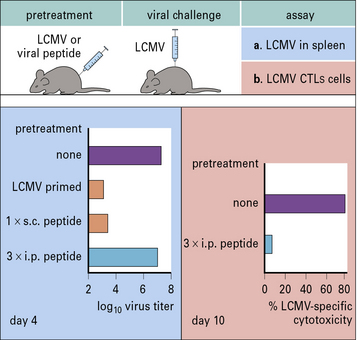
A clear example of how different routes of administration affect the outcome of the immune response is provided by studies of infection with lymphocytic choriomeningitis virus (LCMV). Mice primed subcutaneously with peptide in incomplete Freund’s adjuvant develop immunity to LCMV. However, if the same peptide is repeatedly injected intraperitoneally the animal becomes tolerized and cannot clear the virus (Fig. 11.3).
Regulation by the antigen presenting cell
Neonatal animals are more susceptible to tolerance induction. Therefore mice administered MBP in incomplete Freund’s adjuvant during the neonatal period are resistant to the induction of EAE. This is due to the development of a dominant TH2 response (see Fig. 11.1). The prior TH2 response to MBP prevents the development of the TH1/TH17 pathological response, which mediates EAE. This effect is not restricted to neonatal animals. Indeed, adult Lewis rats can be tolerized to the induction of EAE by similar administration of MBP in incomplete Freund’s adjuvant.
Cytokine production by APCs influences T cell responses
• numerous effects on cell phenotypes; and
• the ability to regulate the type of immune response generated and its extent.
Q. Aside from driving effector T cell responses, how else may APCs affect T cell responses?
A. Dendritic cells or macrophages may produce cytokines such as TGFβ and IL-10 in response to some antigens. These cytokines can drive the conversion of naive CD4+ T cells into regulatory T cells (see Fig. 11.8), which can act as suppressors.
T cell regulation of the immune response
Differentiation into CD4+ TH subsets is an important step in selecting effector functions
• the sites of antigen presentation;
• co-stimulatory molecules involved in cognate cellular interactions;
• peptide density and binding affinity – high MHC class II peptide density favors TH1 or TH17, low densities favor TH2;
• APCs and the cytokines they produce;
• the cytokine profile and balance of cytokines evoked by antigen;
• receptors expressed on the T cell;
• activity of co-stimulatory molecules and hormones present in the local environment;
The cytokine balance controls T cell differentiation
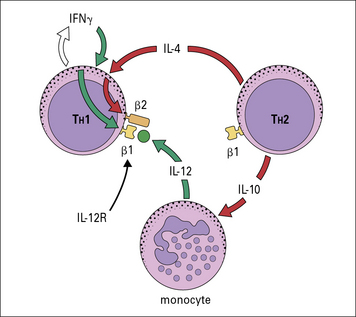
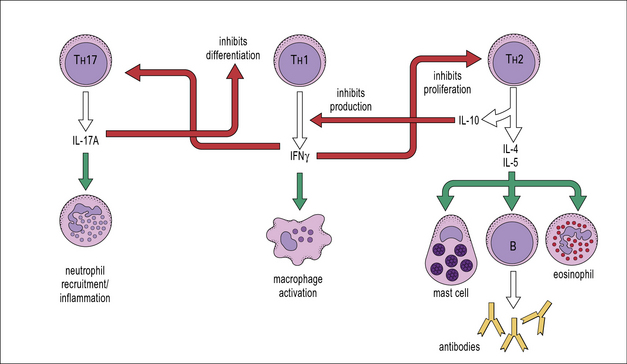
Cytokines from the various TH subsets can cross-regulate each other’s development. Thus, cross-regulation of TH subsets has been demonstrated whereby IFNγ secreted by TH1 cells can inhibit the responsiveness of TH2 cells; also IL-17A can inhibit the development of TH1 responses (Fig. 11.4) whereas IL-10 produced by TH2 cells downregulates B7 and IL-12 expression by APCs, which in turn inhibits TH1 activation.
The TH subset balance is modulated not only by the level of expression of cytokines such as IL-12 or IL-4, but also by expression of cytokine receptors. For instance, the high-affinity IL-12R is composed of two chains, β1 and β2, with both chains being constitutively expressed on TH1 cells. TH1, TH2, and TH17 cells express the β1 chain, but expression of the β2 chain is induced by IFNγ and inhibited by IL-4 (Fig. 11.5). Therefore cytokines reinforce the lineage decisions of the various TH subsets at least in part by controlling the expression of lineage specific receptors.
Q. Apart from the cross-regulation by cytokines described above, in what other ways can a TH1- or TH2-type immune response reinforce itself?
A. The chemokines induced by TH1 and TH2 cells tend to induce the accumulation of the same sets of lymphocytes and their associated effector cells (see Chapter 6).![]()
T cell plasticity
Recently, studies of TH cells have revealed considerable developmental plasticity. In particular, TH17 cells can be induced to produce IFNγ (a TH1 cytokine) and shut down production of IL-17A if they are stimulated in the presence of IL-12 (Fig. 11.w1). Similarly, at least in vitro, TH2 cells can be driven to a mixed TH2/1 phenotype by culture with type one IFNs and IL-12, where cells co-express IL-4 and IFNγ. The reasons for this flexibility in cytokine production remains to be further explored, but it is thought that such plasticity may enable rapid site-specific immune responses to infections to occur.
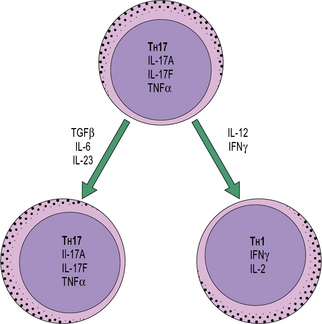
Fig. 11.w1 TH17 differentiation
TH17 cells can differentiate into TH1 cells, depending on the local cytokine milieu.
TH cell subsets determine the type of immune response
• local patterns of cytokine and hormone expression help to select lymphocyte effector mechanism; and
• the polarized responses of CD4+ TH cells are based on their profile of cytokine secretion (Fig. 11.6).
TH1 cytokines including IFNγ, TNFβ, and IL-2 also promote:
TH2 clones are typified by production of IL-4, IL-5, IL-9, IL-10 and IL-13 (see Fig. 11.4). These cells provide optimal help for humoral immune responses biased towards:
• IgG1 and IgE isotype switching;
• stimulation of mast cells, eosinophil growth and differentiation; and
• IL-17A has been proposed to be important for the recruitment of neutrophils and induction of anti-microbial peptides from resident cells.
• IL-17A is important for host defence against Klebsiella pneumonia and Candida albicans.