For in-transit melanoma confined to the extremities, regional chemotherapy in the form of hyperthermic isolated limb perfusion and isolated limb infusion are effective treatment modalities carrying superior response rates to current standard systemic therapy. Despite high response rates, most patients will eventually recur, supporting the role for novel research aimed at improving durable responses and minimizing toxicity. Although the standard cytotoxic agent for regional chemotherapy is melphalan, alternative agents such as temozolomide are currently being tested, with promising preliminary results. Current strategies for improving chemosensitivity to regional chemotherapy are aimed at overcoming classic resistance mechanisms such as drug metabolism and DNA repair, increasing drug delivery, inhibiting tumor-specific angiogenesis, and decreasing the apoptotic threshold of melanoma cells. Concurrent with development and testing of these agents, genomic profiling and biomolecular analysis of acquired tumor tissue may define patterns of tumor resistance and sensitivity from which personalized treatment may be tailored to optimize efficacy. In this article rational strategies for treatment of in-transit melanoma are outlined, with special emphasis on current translational and clinical research efforts.
Epidemiology
While the incidence of several other cancers declines, the incidence of melanoma continues to increase and is now the most common fatal malignancy of young adults, and overall the sixth most common cancer amongst Americans. In fact, an estimated 1 in 50 people will be diagnosed with melanoma over the course of their lifetime. In 2009, there were an estimated 68,720 people newly diagnosed with invasive melanoma, and more than 8650 people died of melanoma in the United States. Unfortunately, mortality rates from melanoma have remained stable because overall poor response of patients with metastatic disease to systemic therapy.
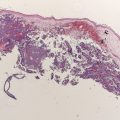
In-transit metastases represent multifocal metastases that spread through the lymphatic system and occur between the site of the primary lesion and the regional draining lymph node basin ( Fig. 1 ). Recently, the 2009 American Joint Committee on Cancer (AJCC) Cancer Staging Manual was published, which recommended the retention of the previous (2002) edition’s staging definition for the in-transit metastases. Patients with in-transit metastases are classified as stage IIIB or IIIC, depending on their regional lymph node status (B = negative, C = positive), regardless of the number of lesions. The number of patients that develop in-transit metastases is not insignificant, with a 30-year German study showing 21% of recurrences are in the form of in-transit or satellite metastases. Another study reports that 2% to 10% of initially treated melanomas of the extremity recur in an in-transit fashion. Historically, this pattern of recurrence is associated with an unfavorable prognosis, with 5-year survival rates ranging from 25% to 30%. However, patients with IIIB in-transit disease seem to have better survival when compared with the rest of their cohort. In the minority of patients, surgical excision of in-transit metastases can be used when the in-transit disease is limited to a few tumor deposits. Unfortunately, the majority of patients have multifocal disease for which standard of care systemic chemotherapy or immunotherapy has had limited benefit. However, if patients have in-transit disease confined to the extremities, regional chemotherapy delivered by isolated limb perfusion or isolated limb infusion is an effective treatment option associated with complete response rates ranging from 23% to 82% ( Table 1 ). Regional chemotherapy for in-transit disease is a rapidly progressing field and is a platform for ongoing research aimed at delineating underlying melanoma tumor biology. This review describes the current status of regional chemotherapy in treating patients with in-transit disease of the extremity, and the novel approaches being developed using targeted agents and immune modulators in an effort to improve efficacy while minimizing toxicity.

| Study | N | CR (%) | PR (%) | SD (%) | PD (%) | Condition | |
|---|---|---|---|---|---|---|---|
| HILP | Minor et al, 1985 | 18 | 82 | 18 | 0 | 0 | Hyperthermia |
| Storm and Morton, 1985 | 26 | 50 | 31 | 19 a | 0 | Hyperthermia | |
| Kroon et al, 1987 | 18 | 38 | 44 | 17 a | 0 | Normothermia | |
| Kroon et al, 1993 | 43 | 77 | 14 | 9 a | 0 | Normothermia | |
| Klaase et al, 1994 | 120 | 54 | 25 | 21 a | 0 | Normothermia | |
| Grünhagen et al, 2004 | 100 | 69 | 26 | 5 a | 0 | Hyperthermia | |
| Aloia et al, 2005 | 59 | 57 | 31 | 12 a | 0 | Hyperthermia | |
| Cornett et al, 2006 | 58 | 25 | 39 | 28 | 11 | Hyperthermia | |
| Sanki et al, 2007 | 120 | 69 | 16 | 0 | 15 | Hyperthermia | |
| ILI | Mian et al, 2001 | 9 | 44 | 56 | 0 | 0 | |
| Lindner et al, 2002 | 128 | 41 | 44 | 12 | 4 | ||
| Bonenkamp et al, 2004 | 13 | 31 | 61 | 0 | 8 | ||
| Brady et al, 2006 b | 22 | 23 | 27 | 0 | 50 | ||
| Beasley et al, 2008 | 50 | 30 | 14 | 10 | 46 | ||
| Kroon et al, 2008 c | 185 | 38 | 46 | 10 | 6 | ||
| Beasley et al, 2009 | 128 | 31 | 33 | 7 | 29 |
b Includes 1 patient with advanced sarcoma.
Isolated limb perfusion
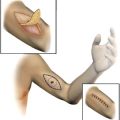
Regional treatment, in the form of hyperthermic isolated limb perfusion (HILP), was first performed for in-transit melanoma more than 50 years ago by Creech and colleagues. This technique is still used today and overall remains unchanged from its original components. In brief, the femoral or subclavian vessels are surgically exposed and cannulated. When indicated, lymphadenectomy can also be performed during the vascular exposure. The artery and vein are cannulated at the root of the limb and an Esmarch tourniquet is placed proximal to the cannulated vessels. Perfusion proceeds with the use of a high-flow, melphalan-based perfusate, using a membrane oxygenator to maintain the acid-base status and oxygenation of the isolated limb in the physiologic range ( Fig. 2 ). Creech and colleagues used melphalan as their chemotherapeutic agent based on in vivo mouse data, and this has remained the standard agent for isolated limb perfusion (ILP). Hyperthermia of the limb is achieved due to the heated, high-flow perfusate, as well as warming blankets wrapped around the extremity for the duration of the procedure.
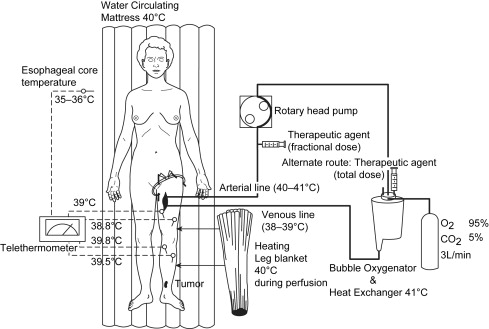
Retrospective studies have shown up to 82% of patients experience a complete response after ILP depending on the patient population and particular adjuncts, but larger studies seem to demonstrate complete response rates in the 50% to 70% range (see Table 1 ). For instance, the Sydney Melanoma Unit (SMU) has reported an overall response rate of 75%, with 69% of patients experiencing a complete response when treated with ILP with regional melphalan ± actinomycin D or regional cisplatin. In the authors’ Duke University experience of melphalan-based ILP, 88% of patients responded and 57% were complete responders. One of the larger series by Grunhagen and colleagues reported an overall response rate of 95%, with 69% complete responders who received HILP with melphalan and adjunctive tumor necrosis factor-α (TNF-α). The overall 5-year survival rate for this cohort was 32%; the median survival was 25 months.
Isolated limb perfusion
Regional treatment, in the form of hyperthermic isolated limb perfusion (HILP), was first performed for in-transit melanoma more than 50 years ago by Creech and colleagues. This technique is still used today and overall remains unchanged from its original components. In brief, the femoral or subclavian vessels are surgically exposed and cannulated. When indicated, lymphadenectomy can also be performed during the vascular exposure. The artery and vein are cannulated at the root of the limb and an Esmarch tourniquet is placed proximal to the cannulated vessels. Perfusion proceeds with the use of a high-flow, melphalan-based perfusate, using a membrane oxygenator to maintain the acid-base status and oxygenation of the isolated limb in the physiologic range ( Fig. 2 ). Creech and colleagues used melphalan as their chemotherapeutic agent based on in vivo mouse data, and this has remained the standard agent for isolated limb perfusion (ILP). Hyperthermia of the limb is achieved due to the heated, high-flow perfusate, as well as warming blankets wrapped around the extremity for the duration of the procedure.
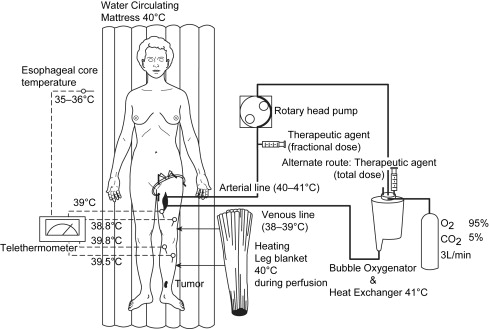
Retrospective studies have shown up to 82% of patients experience a complete response after ILP depending on the patient population and particular adjuncts, but larger studies seem to demonstrate complete response rates in the 50% to 70% range (see Table 1 ). For instance, the Sydney Melanoma Unit (SMU) has reported an overall response rate of 75%, with 69% of patients experiencing a complete response when treated with ILP with regional melphalan ± actinomycin D or regional cisplatin. In the authors’ Duke University experience of melphalan-based ILP, 88% of patients responded and 57% were complete responders. One of the larger series by Grunhagen and colleagues reported an overall response rate of 95%, with 69% complete responders who received HILP with melphalan and adjunctive tumor necrosis factor-α (TNF-α). The overall 5-year survival rate for this cohort was 32%; the median survival was 25 months.
Isolated limb infusion
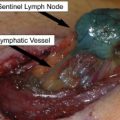
More recently, Thompson and colleagues at the SMU developed an alternative to HILP, called isolated limb infusion (ILI). ILI is less invasive than HILP, as it is performed via percutaneous catheterization of the involved limb. Using the Seldinger technique under fluoroscopic guidance, arterial and venous catheters are placed into the involved limb ( Fig. 3 ). A pneumatic or Esmarch tourniquet is then positioned at the most proximal portion of the limb and inflated, thereby isolating the limb from systemic circulation. The extremity is wrapped with warming blankets using circulated heated water for the duration of the procedure. Next, melphalan is rapidly infused into the arterial catheter and manually circulated through a blood warmer syringe and a 3-way stopcock. After circulating for 30 minutes, a washout procedure using crystalloid fluids removes the chemotherapy from the limb via venous outflow extraction.
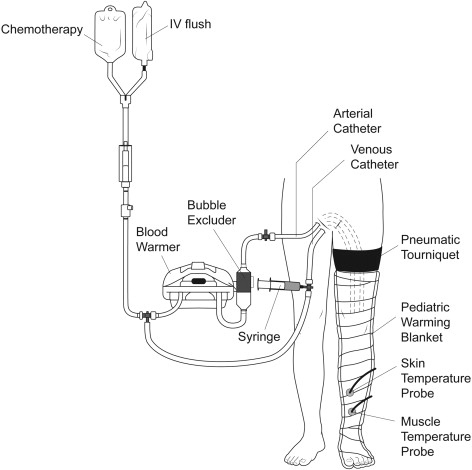
In contrast to ILP, ILI is a low-flow circuit with no oxygenator, resulting in the limb becoming normothermic, hypoxic, and acidotic. It is postulated that the limb acidosis and hypoxia may increase melphalan activity. The simplicity of ILI has several advantages over traditional HILP. First of all, it does not require a membrane oxygenator or pump priming with blood. It is also a shorter procedure, is repeatable, and is associated with less regional toxicity when correcting for ideal body weight. ILI is the preferred treatment for more frail patients with multiple comorbidities who may not tolerate the more involved HILP procedure. To be fair, there are also potential disadvantages of ILI. Namely, the same degree of hyperthermia as HILP cannot be routinely achieved by ILI. Furthermore, ILI uses a lower dose of melphalan, and its duration is half as long as that with HILP. These differences probably contribute to the lower overall response rates of ILI as compared with HILP in a recent multicenter combined analysis (79% in 294 patients vs 64% in 313 patients). While low- to moderate-grade toxicities are similar for both ILI and HILP, HILP appears to be associated with more treatment-related limb loss.
Regional chemotherapy
The major difference between systemic and regional chemotherapy treatments is the ability to deliver very high doses of chemotherapy through an isolated extremity circuit while minimizing systemic leakage and resultant systemic toxicity. Because the typical leak rate is less than 1% when performing HILP/ILI with conventional cytotoxic drugs, there are few systemic side effects and organ toxicity is rarely dose-limiting. As a result, plasma melphalan can safely reach levels 10- to 100-fold times higher following regional compared with systemically delivered chemotherapy. Drug delivery is also enhanced through its local delivery and avoidance of hepatic metabolism and renal clearance. Adjuvant treatments, such as hyperthermia, may affect the pharmacokinetics of the chemotherapeutic agent, and can be applied more safely and effectively to the extremity for a regional treatment as compared with systemic chemotherapy. HILP/ILI are also unique compared with systemic therapy in that after chemotherapy has circulated in the extremity, a washout procedure eliminates the remaining drug, reducing the risk of systemic toxicity before the tourniquet is released. Melphalan remains the most common agent used in regional chemotherapy for melanoma, and is considered the standard of care for the procedures. However, alternative agents including cisplatin and temozolomide may offer significant advantages over melphalan in certain circumstances.
Melphalan
The standard chemotherapy agent used in regional chemotherapy for melanoma is melphalan ( l -phenylalanine mustard [LPAM]). LPAM is a bifunctional alkylating agent, and remains the most common drug used for HILP/ILI procedures. The rationale in using melphalan stems from the essential role phenylalanine plays in melanin synthesis. In theory, the phenylalanine derivative melphalan should provide selective toxicity to melanocytes and melanoma cells in which melanin is synthesized. Despite its theoretical selective toxicity for metastatic melanoma, systemic melphalan is relatively ineffective because the maximally tolerated dose is much lower than the effective dose. This limitation is overcome with regional delivery techniques, because much higher doses can be achieved in the extremity than would be tolerated when administered systemically.
Regional melphalan dosing is based in part on the report by Roberts and colleagues, who determined that treatment responses tend to plateau at achieved melphalan levels of 25 μg/mL. Melphalan concentrations above this value are routinely achieved by HILP/ILI, so increasing the dose of melphalan to achieve levels higher than this is not likely to affect the response rate and may only increase toxicity. Properties of melphalan that make it particularly effective as a regionally delivered chemotherapy include its short half-life, limited cell cycle specificity, low vascular endothelium and soft tissue toxicity, and linear dose-response relationship with respect to cytotoxicity.
Melphalan has historically been dosed and administered based on body weight over a wide dose range (0.25–2.8 g/kg) without accounting for body mass distribution. Dosing is now based on limb volume, with a target melphalan dose of 7.5 mg/L and 10 mg/L for a lower extremity ILI and HILP, respectively, and 10 mg/L and 13 mg/L for an upper extremity ILI and HILP, respectively. Based on experience, the authors have recommended a total dose not exceeding 100 mg for the lower extremity or 50 mg for the upper extremity, although there have been reports of maximum doses as high as 120 mg for the lower extremity and 80 mg for the upper extremity.
Limb volume can be estimated using volume displacement or limb circumference. Volume displacement may offer a more accurate estimation; however, it can be more cumbersome and may necessitate the position of the tourniquet be predetermined. The authors’ preferred method is using the limb circumference measurement by which the volume of the limb is calculated based of serial circumference measurements (eg, every 1.5 cm). Despite this being a calculated estimate rather than a direct measurement, it allows the volume to be determined in the clinic or even during the procedure once the final position of the tourniquet has been decided. The authors find this method to be more flexible by facilitating a more real-time calculation of limb volume at the time of the actual procedure. If the hand or foot of the involved extremity is free of disease, a second optional Esmarch or pneumatic tourniquet may be used to exclude these areas from the circuit. In this situation, the hand or foot volume should be subtracted from the total limb volume. Some groups, including the authors’ own group, now correct melphalan dosing for ideal body weight based on evidence that this dose modification is associated with lower toxicity without altering the complete response rate.
Cisplatin
Cisplatin is an attractive agent, as it is commonly used systemically for the treatment of melanoma. By cross-linking DNA, cisplatin interferes with mitosis, leading to eventual apoptosis. Essential to its utility as a regional chemotherapy agent, cisplatin does not require metabolic transformation to become an active antineoplastic agent and it is cell-cycle independent. Indeed, initial animal studies using cisplatin as a regional chemotherapy agent were promising and were quickly translated into clinical trials. Unfortunately, these subsequent clinical studies have produced mixed results.
Early, small clinical studies reported favorable response rates after regional cisplatin perfusion without significant toxicities. In a larger series of 58 melanoma patients reported by Hajarizadeh and colleagues, 41 patients received prophylactic HILPs with cisplatin for stage I disease and 17 others were treated for stage II, III, or IV disease. In this study, HILP was followed by wide local excision of the primary tumor or reexcision of any remaining melanoma in all patients. After a median follow-up of 29 months, local recurrence rates of 12%, 33%, and 30% were observed for stage I, II, and III disease, respectively. Unfortunately, significant complications occurred in 8 patients, leaving 2 with permanent deficits. This study concluded that HILP with cisplatin was more effective than surgery alone to achieve local control and in the short-term appeared to be at least as effective as HILP with melphalan.
However, subsequent studies have shown disappointing results for HILP with cisplatin both in terms of efficacy and toxicity. Santinami and colleagues discontinued a study over concerns about toxicity after only 9 patients with melanoma had been treated. In a phase 1 trial of 15 patients of HILP with cisplatin, Coit and colleagues reported only 3 patients having a complete remission lasting more than 2 years as well as 3 patients developing severe limb-threatening toxicity. These investigators eventually concluded that HILP with cisplatin was not justified as a standard therapy for metastatic in-transit melanoma. Similarly, Thompson and Gianoutsos suggested cisplatin was not the drug of choice for HILP treatment of melanoma confined to the limb when 5 of 6 patients receiving therapeutic HILPs with cisplatin did not achieve complete responses and 2 of 4 patients receiving prophylactic treatments developed early recurrence. Confounding these results, toxicity was very significant, resulting in an amputation in one patient and leaving another with a permanent foot drop. In the patient who had the leg amputation, melanoma remained despite extensive necrosis of normal tissue. At present, cisplatin is not used because of the incidence of complications and the lack of a significant improvement in response over melphalan.
Temozolomide
Temozolomide (TMZ) is considered to have some activity for metastatic melanoma as an oral agent. A new intravenous (IV) formulation of temozolomide has been developed, and appears to hold tremendous potential for use in regional chemotherapeutic treatments. Temozolomide is an alkylating agent that is metabolized to the active metabolite, methyldiazonium ion (MTIC), which methylates guanine residues in DNA at the O 6 and N 7 positions. Cellular apoptotic pathways become activated when DNA mismatch enzymes attempt to repair the O 6 -methylguanine generated by temozolomide, resulting in single- and double-stranded DNA breaks. Temozolomide is suitable to the regional model because unlike dacarbazine (DTIC), temozolomide has 100% bioavailability under physiologic conditions and does not require hepatic conversion. Preclinical in vivo studies in nude rats implanted with melanoma xenografts demonstrated that regional infusion with temozolomide resulted in prolonged tumor growth delay compared with rats that received systemic temozolomide or regional melphalan, especially in tumors with low O 6 -alkylguanine-DNA alkyltransferase (AGT) activity, the predominant mechanism of resistance of temozolomide. Analysis across a panel of xenografts suggested that approximately 20% of tumors preferentially respond to temozolomide, 20% preferentially respond to regional melphalan, and 60% respond equally to temozolomide or melphalan. The Food and Drug Administration (FDA) recently approved the IV formulation of temozolomide; a phase 1 clinical trial is under way at Duke University, The University of Texas MD Anderson (MDACC), and Moffitt Cancer Center to define the toxicity profile and maximally tolerated dose to be used in regional therapy.
Algorithm
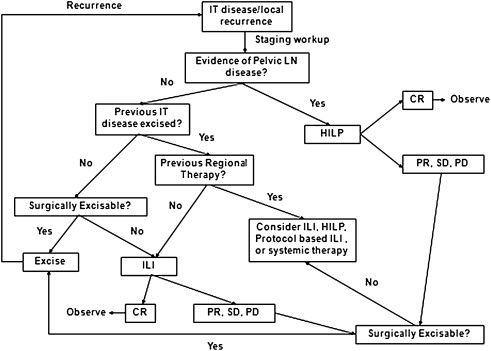
Despite the frequency with which HILP and ILI are performed for in-transit melanoma, no consensus exists as to which treatment is preferable or what strategies surgeons should follow should a patient progress after regional treatment. In general, the authors perform surgical excision for small volume disease, usually defined as 1 to 2 lesions that can be resected with negative surgical margins. Often, in patients who undergo surgical excision the authors will also perform a simultaneous sentinel lymph node biopsy, which reports suggest will be positive about 50% of the time. Regional therapy is used in patients who have failed surgical excision, have nonresectable extremity disease, or have multifocal disease of 3 or more lesions. The authors do not recommend excision of lesions in the field of treatment if patients are undergoing regional therapy. The rationale for this twofold: (1) potential wound-healing issues related to the chemotherapy that can cause limb swelling and tissue damage, and (2) leaving the lesions in place provides an opportunity for the surgeon to monitor the effectiveness of the treatment.
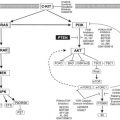
The algorithm for which type of regional treatment (HILP or ILI) the authors use is as follows. Patients with local recurrence or with evidence of pelvic, femoral, or axillary nodal involvement may be candidates for HILP, as excision of the tumor-containing lymph nodes exposes the vessels used for this procedure. For patients with femoral or axillary nodal involvement, a less invasive alternative is to treat these patients with an ILI, and then perform the lymphadenectomy at the end of the ILI once the heparinization is reversed and after the infusion catheters are removed. Some centers may preferentially offer ILP as the initial treatment to patients with high disease burden, as these patients may be more likely to have regional nodal disease. However, there are no strong data suggesting that patients with high disease burden are more likely to respond to HILP as compared with ILI. The authors’ group has favored using ILI first, even in patients with high disease burden, leaving HILP as a potential salvage therapy for those who do not respond to ILI. In addition, there are several protocol-based ILI trials (such as temozolomide, or melphalan in combination with other targeted agents), or systemic therapy ( Fig. 4 ) for patients who either fail regional treatment or progress.
Strategies to optimize response
As highlighted earlier, overall and complete response rates, as well as durability of response, are variable depending on the regional chemotherapy platform as well adjunctive therapies. In the context of regional chemotherapy, standard adjuncts to optimize therapy include the addition of hyperthermia and TNF-α to ILP. Newer strategies under active investigation include novel agents that overcome traditional drug resistance mechanisms, target tumor vasculature, and lower the apoptotic threshold of malignant melanoma.
Hyperthermia
Hyperthermia may augment or sensitize melanoma to cytotoxic therapy by increasing blood flow, membrane permeability, local metabolism, and drug uptake. Indeed, hyperthermia continues to be an essential component of ILP since its introduction by Cavaliere in 1967. Based on preclinical experiments suggesting heat to be tumoricidal in a rat melanoma model, Cavaliere and colleagues first explored the effects of heat alone in 22 patients with recurrent extremity tumors. The morbidity of the procedure was quite high, with 6 immediate postoperative deaths. Of the remaining 16, 12 patients were alive without evidence of disease at 3 to 28 months. Two years later, Stehlin combined regional chemotherapy with heated perfusion, and established HILP in a reported experience of 37 patients. Of the 37 melanoma patients, only 12 were evaluated for measurable disease. Of these, 10 of the 12 patients (83%) had a response described as “pronounced regression” for greater than 3 months. Of note, this report used very high perfusate temperatures reaching up to 46°C. As a modern comparison, the authors initiate HILP at 38.5°C with a goal temperature of 40°C.
In 1989 the first prospective, randomized trial was conducted to evaluate the role of hyperthermia and regional extremity perfusion of in-transit melanoma. In this study, 107 patients were randomized to receive either surgical excision or surgical excision followed by HILP with melphalan. With a primary end point of disease-free survival, the study was stopped prematurely (median follow-up 550 days) due to a highly significant advantage in disease-free survival in the HILP arm (89% vs 52%). In addition, there was an improvement in overall survival (98% vs 86%), with minimal local and systemic complications.
There are also strong animal model data in the context of regional therapy with the alkylating agents melphalan and temozolomide suggesting that hyperthermia augments their antitumor efficacy against both melanoma and sarcomas. To date, no randomized controlled trial has compared hyperthermic ILP with normothermic ILP. Despite lacking level I evidence, hyperthermia remains a standard component of ILP because of the strong indirect clinical evidence and theoretical merit.
Tumor Necrosis Factor-α
The first study of TNF-α in a human trial of HILP was performed by Posner and colleagues in 1995. In this study of 6 patients receiving escalating doses of TNF-α alone during HILP, only 1 patient had a complete response. A subsequent study combined TNF-α with interferon-γ (IFN-γ) and melphalan delivered via HILP. Interferon was added for potential antitumor synergism. Overall, this treatment strategy was quite toxic: all patients required dopamine infusions for a severe inflammatory response, and due to limb toxicity 2 patients even required an amputation. Despite the toxicity, 89% of patients had a complete response and 11% had a partial response to the combination treatment at 11 months’ follow-up.
Based on this study, interest in TNF-α in addition to HILP began to increase, eventually resulting in a randomized trial of 103 patients conducted by the American College of Surgeons Oncology Group (ACOSOG). This trial was stopped early after an interim analysis showed no evidence of improved patient responses, with significant increases in severe toxicities in the TNF-α plus melphalan arm. At 6 months, although there remained no significant improvement with the addition of TNF-α in the 89 patients still in the study, a difference in response rates was noted (42% complete response with combination vs 20% in single therapy; P = .101). Critics of this trial have cited an early time point for assessing response (3 months) as the reason for the lower response rates observed with TNF-α compared with prior trials. However, a recent large single-institution series from the United States has also failed to demonstrate an improvement in regional in-field progression-free survival with the use of TNF-α. Due to the inability to clearly demonstrate any benefit from the addition of TNF to regional therapy, it has not been FDA-approved as an adjunct for HILP in the United States.
Targeting Traditional Drug Resistance Mechanisms
Drug metabolism
One mechanism of melanoma chemoresistance is hypermetabolism of alkylating chemotherapy agents by glutathione- S -transferases (GSTs). GSTs are a family of phase II detoxification enzymes that catalyze glutathione (GSH) to electrophiles including carcinogens, mutagens, and anticancer reagents. Melanoma cells as well as tumor types have been shown to express elevated GST and GSH levels, which in turn have been associated with resistance to chemotherapy. Further supporting its critical role in tumor chemoresistance, melphalan exposure appears to increase tumor GSH levels by more than two-thirds. Conversely, reducing GSH levels confers or restores sensitivity in vitro. The GST enzymes, which are polymorphic and exist in both membrane-bound and cytosolic forms, are divided into 8 classes: Alpha, Kappa, Mu, Pi, Theta, Sigma, Omega, and Zeta. Through conjugation of GSH to intracellular melphalan, GSTs effectively neutralize melphalan by inhibiting access to DNA by the alkylating agent. Given their great potential to augment the chemosensitivity of melanoma as well as other tumor types, there exists significant interest in developing novel inhibitors of GST-mediated metabolism.
One strategy in overcoming GST-mediated drug metabolism is to deplete the tumor cells of available GSH, thereby preventing conjugation of the GSH by GSTs to alkylating chemotherapy agents. l – S,R -Buthionine sulfoximine (BSO) is a potent and specific inhibitor of the enzyme γ-glutamylcysteine synthetase (GCS), which is the rate-limiting step in the production of GSH. BSO has been shown to decrease intracellular GSH levels, increase DNA cross-linking, and sensitize the cells to cytotoxicity. Early, small clinical studies of continuous BSO infusion with intravenous melphalan were limited by occasional severe myelosuppression. While the addition of BSO to systemic melphalan may be associated with myelosuppression, one might predict that the combination of BSO and melphalan in regional therapy would not be limited by systemic toxicity. In a nude rat xenograft model of ILI, systemic BSO treatment reduced resistance to ILI melphalan. Intraperitoneal administration of BSO was well tolerated and was associated with an approximately 72% reduction in tumor GSH levels. BSO treatment was associated with an increase in tumor growth delay relative to saline and melphalan-alone controls. Based on these studies, a phase 1 trial at Duke University using a 3-day infusion of BSO around the time of melphalan ILI was opened for enrollment, but was closed prematurely due to the restricted availability of the intravenous formulation of BSO.
One of the more promising targets for inhibiting the GST pathway drug metabolism is enzyme glutathione- S -transferase P1 (GSTP1). In addition to its metabolizing function, GSTP1 also serves as a major regulator of cell signaling in response to stress, hypoxia, growth factors, and other stimuli. GSTP1 inhibits downstream mitogen-activated protein (MAP) kinase signaling mediated by binding c-Jun N-terminal kinase (JNK), thereby preventing the phosphorylation of c-Jun. The novel GSTP1 inhibitor 6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio)hexanol (NBDHEX) has recently been shown to inhibit JNK pathway activation, leading to melanoma cell apoptosis in vitro as well as potent inhibition of tumor growth in vivo. The exact role of GSTP1 in melanoma tumorigenesis and its potential for augmenting currently available alkylating chemotherapy agents remains an active area of investigation.
DNA repair
Should temozolomide be shown to be an effective regional chemotherapy agent on completion of phase 1 and 2 trials, strategies to impair melanoma DNA repair induced by this compound will become important. TMZ confers its cytotoxicity by alkylating or methylating DNA at O 6 -guanine, N 7 -guanine, and N 3 -adenine residues. This methylation results in DNA damage and eventual cell death. Temozolomide cytotoxicity can be overcome, however, in tumor cells through several different DNA repair mechanisms including the O 6 -methylguanine-DNA methyltransferase (MGMT), O 6 -alkylguanine-DNA alkyltransferase (AGT), the DNA-mismatch repair system (MMR), and the base excision repair (BER) pathways. Two drugs, the Poly(ADP-ribose) polymerase (PARP) inhibitor INO-1001 and the AGT inhibitor O 6 -benzylguanine ( O 6 -BG) have been tested in a preclinical setting in an effort to delineate the importance of DNA repair inhibition in augmenting regionally delivered temozolomide.
PARP recognizes DNA damage and activates the BER pathway by recruiting the DNA repair proteins XRCC1, DNA ligase III, and DNA polymerase β. Cells without PARP activity have been to shown to be more sensitive to alkylating chemotherapy, demonstrating increased apoptosis and chromosomal instability. Based on these data, the novel PARP inhibitor INO-1001 was tested in a melanoma model to determine whether it could augment the response to temozolomide. Toshimitsu and colleagues recently tested this hypothesis using a rat xenograft model of regional chemotherapy. In this study, systemic administration of the PARP inhibitor INO-1001 followed by regional infusion of temozolomide did not significantly decrease tumor growth in melanoma xenografts with intact mismatch repair mechanisms and high MGMT activity. However, in the xenograft that exhibited deficient mismatch repair without MGMT activity, termed PRMel, systemic INO-1001 followed by regional infusion of temozolomide significantly decreased tumor growth compared with regional temozolomide alone (22.6% vs 322.8% increase in tumor volume at day 40).
Inhibition of the DNA repair enzyme AGT using O 6 -BG has also been tested and shown to reduce tumor AGT activity by 93.5%. Regionally administered temozolomide in conjunction with systemic O 6 -BG led to significant tumor growth delay compared with either regional temozolomide alone or systemic temozolomide plus systemic O 6 -BG. Two of 6 rats had tumor regression in the group treated with 750 mg/kg temozolomide alone versus 6 of 6 rats with regression in the group treated with O 6 -BG for 5 days plus 750 mg/kg temozolomide.
Vascular Targeting Agents
ADH-1
ADH-1 (Exherin) is a cyclic pentapeptide that disrupts N-cadherin binding interactions. The cadherins are a large supergene family of proteins involved in cell-to-cell adhesion. Malignant transformation in melanoma is characterized by a “switch” from E-cadherin to N-cadherin, altering intracellular signaling pathways and leading to increased proliferation, survival, and angiogenesis, as well as decreased apoptosis. In preclinical studies, the use of systemic ADH-1 in conjunction with regional melphalan showed marked increases in tumor responses even in the most melphalan-resistant tumors. Subsequent mechanistic studies suggest that the efficacy of ADH-1 in augmenting tumor responses may result from disrupting tumor cell interactions with its microenvironment. In the rat xenograft ILI model, ADH-1 significantly increased melphalan-DNA adduct formation in tumor cells, suggesting that ADH-1 may augment tumor response to melphalan by increasing delivery of the relatively small melphalan molecule into tumor cells. This finding has been further supported by in vitro and in vivo permeability assays showing increasing permeability of both normal and tumor microvasculature after treatment with ADH-1. The exact role of N-cadherin in melanoma tumor signaling remains unclear, and is an active area of investigation in the authors’ laboratory.
As a direct result of the marked responses seen in preclinical experiments, a phase 1 trial was conducted. Sixteen patients were treated without dose-limiting toxicities using a single dose of ADH-1 prior to ILI and a second dose 1 week after ILI. The ADH-1 was dose-escalated while the ILI was kept at its standard dosing. In-field responses included 8 complete responders and 2 partial responders. Given lack of additional toxicity from ADH-1 and a surprising 50% complete response rate, a multicenter phase 2 trial of systemic ADH-1 in combination with melphalan via ILI for patients who have AJCC Stage IIIB or IIIC extremity melanoma was performed and recently completed. This trial marks the first prospective multicenter (Duke University, MDACC, Moffit Cancer Center, and University of Florida) trial of ILI as well being the first to investigate whether a targeting agent could augment regional chemotherapy responses. In total, 45 patients were enrolled over 15 months at the 4 institutions. ADH-1 was well tolerated without significant additional toxicity. Pretreatment using ADH-1 prior to ILI with melphalan resulted in an increase in overall and complete response rates compared with historical controls as measured at 3 months. However, many of these patients recurred 5 to 6 weeks after the 3-month time point, such that there was no significant difference in regional progression-free survival curves between the study participants and patients treated with ILI alone off this study at the same institutions.
Bevacizumab
Another promising vascular targeting agent for augmenting regional chemotherapy is bevacizumab, a monoclonal antibody to vascular endothelial growth factor (VEGF). Bevacizumab has been used in combination with standard chemotherapies to treat patients with metastatic colorectal, breast, brain, kidney, and lung cancers. The classic mechanism of bevacizumab is sequestration of VEGF and inhibition of aberrant tumor vasculature formation. Moreover, increasing evidence suggests inhibition of VEGF via bevacizumab can augment chemotherapy agents by “normalizing” tumor vasculature, leading to greater delivery of chemotherapy to tumor cells. Bevacizumab is currently being investigated in combination with other chemotherapy agents for metastatic melanoma in multiple clinical trials across the United States. A recent phase 2 study examining bevacizumab in combination with the mammalian target of rapamycin (mTOR) inhibitor everolimus demonstrated moderate activity in 57 patients with metastatic melanoma. When combined with dacarbazine and IFN-α2a in 26 patients, bevacizumab had moderate activity, with an overall response rate of 23% and 2 complete responders. The median overall survival for all patients in this study was 11.5 months. In another phase 2 trial of 53 patients, bevacizumab in combination with paclitaxel and carboplatin was found to be moderately well tolerated, and resulted in 9 (17%) patients achieving partial regression and 30 (57%) achieving stable disease at 8 weeks. Given multiple phase 2 trials suggesting improved overall response rates in combination with systemic chemotherapy as well as its inherent potential to increase drug delivery through tumor vasculature “normalization,” the authors hypothesized that systemically administered bevacizumab prior to regionally delivered melphalan would significantly enhance the efficacy of regionally delivered melphalan. Indeed, preclinical evidence in a rat melanoma xenograft model suggests that a single injection of bevacizumab given 3 days before ILI with melphalan markedly improved response rates (manuscript in preparation). Tumors pretreated with bevacizumab demonstrated decreased tumor vasculature density and permeability, yet had increased melphalan-DNA adduct formation. Taken together, these studies support the concept that bevacizumab results in tumor vascular normalization, thereby enhancing drug delivery and improving overall response to regional chemotherapy (manuscript in preparation). A phase 1 trial combining bevacizumab with melphalan via ILI is currently being planned at Duke University for 2011.
Altering Apoptotic Threshold
Sorafenib
Altered cellular signaling cascades in melanoma have been shown to increase proliferation and decrease apoptosis, leading to greater chemoresistance. One of the more common oncogenic pathways altered in melanoma is the BRaf serine/threonine kinase pathway. In fact, 50% to 70% of melanomas express the constitutively active mutant BRaf serine/threonine kinase. Although more than 30 mutations of the BRAF gene have been identified and associated with malignancy, the most frequent mutation (∼90%) is a single substitution of glutamine for valine at residue 599, which is referred to as V600E. The end effect of this mutation is transformation of the BRaf kinase from the inactive to constitutionally active state, resulting in continuous stimulation of MAP kinase and extracellular regulated (ERK) kinase pathways with resultant augmentation of proliferation, differentiation, angiogenesis, and apoptosis. Given the high incidence of BRAF mutation in melanoma and its oncogenic effects, the BRaf kinase is a promising target for enhancing systemic as well as regional chemotherapy.
Sorafenib (Nexavar) is a commercially available drug approved for the treatment of renal cell and hepatocellular carcinomas. This small molecule inhibits multiple tyrosine kinase pathways including the VEGF receptor-2 and -3 kinases, c-Kit, and the MAP kinases pathways, including BRaf. Through its inhibition of the Raf-MEK-ERK pathway, sorafenib has been shown to inhibit cell proliferation and angiogenesis as well as to induce apoptosis by downregulating the antiapoptotic protein Mcl-1 and preventing the nuclear translocation of apoptosis-inducing factor (AIF). Although effective in preclinical in vitro and in vivo models, clinical trials with sorafenib as a single agent in melanoma have produced disappointing results. However, subsequent phase-1 to -2 trials showed greater overall tumor response rates when sorafenib was combined with cytotoxic therapies including carboplatin and paclitaxel, temozolomide, dacarbazine, or IFN-α2a. Unfortunately, in a phase 3 randomized, controlled trial, the combination of sorafenib with carboplatin plus paclitaxel for patients with advanced melanoma failed to demonstrate improvements in any clinical end points. Although sorafenib failed to synergistically enhance systemically administered cytotoxic therapy, whether it could augment regionally delivered chemotherapy whereby dose of chemotherapy can be an order of magnitude higher remained unclear.
In preclinical studies, sorafenib significantly augmented melanoma chemosensitivity to melphalan and temozolomide across a panel of 24 cell lines independent of BRaf or NRas mutational status. Using a rat xenograft model of ILI, the combination of sorafenib and either melphalan or temozolomide significantly reduced tumor growth after ILI as compared with melphalan or temozolomide alone. On immunohistochemical analysis of tumor tissue acquired 24 hours after ILI, rats pretreated with sorafenib exhibited increased apoptosis, with associated decreases in ERK activation and Mcl-1 protein levels. Overall, the results of these preclinical studies suggested that sorafenib reduces tumor apoptotic threshold and thereby sensitizes melanoma tumor cells to the cytotoxic agents melphalan and temozolomide. These promising preclinical results led to an open-label, multicenter, phase 1 dose escalation study to evaluate safety, tolerability, and antitumor activity of oral sorafenib in combination with normothermic ILI with melphalan at Duke University and Memorial Sloan-Kettering Cancer Center. This study has been recently completed and involved 20 patients. From this trial, a maximally tolerated oral dose of sorafenib was defined as 200 mg twice daily. Overall response and complete response rates in this small study did not exceed historical measures of standard melphalan ILI alone, and patients pretreated with higher doses of sorafenib did seem to have an increased risk of local toxicity (ie, ulceration and myositis) without an obvious improvement in response rates. Of note, preliminary analyses have not associated response to sorafenib and melphalan with BRaf or N-ras mutational status. Final analyses of this trial are ongoing and are soon to be published.
Dasatinib
Another potential target in melanoma is the src family of kinases. Src kinase overexpression leads to increased cellular proliferation and decreased adhesion. Src signals through its downstream intermediaries, called signal transducers and activators of transcription (STAT) factors. One of these factors, STAT3, has been found to be activated in the majority (85%) of melanoma cell lines. STAT3 has been shown to not only promote tumor growth and survival, but also appears to upregulate VEGF and promote tumor angiogenesis. Inhibition of src kinase blocked the growth of human melanoma cell lines with elevated STAT3 activity and induced apoptosis by inhibiting the expression of anti-apoptotic genes Bcl-x L and Mcl-1.
By mitigating the unchecked STAT3 activity leading to expression of prosurvival genes, Src inhibitors such as dasatinib (Sprycel) may also potentiate the effectiveness of traditional chemotherapy by lowering the apoptotic threshold. Dasatinib is manufactured by Bristol-Myers Squibb, and is a BCR-Abl and Src family tyrosine kinase inhibitor currently approved for the treatment of chronic myelogenous leukemia refractory to imatinib treatment, as well as acute lymphoblastic leukemia positive for the Philadelphia chromosome. Recent in vitro studies of have shown dasatinib to synergistically enhance the effects of cisplatin, but not temozolomide or paclitaxel in melanoma. In regard of the role of dasatinib in augmenting regional chemotherapy for in-transit melanoma, preclinical studies are currently ongoing and promising. Using a nude rat xenografts model of ILI, rats pretreated with orally administered dasatinib followed by regionally delivered melphalan have shown superior tumor responses compared with rats receiving regional melphalan therapy alone (unpublished data). Based on these preliminary data, the authors are planning for a phase 1 clinical trial for melanoma patients with in-transit disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree