Regional cancer therapies are treatments that direct a therapeutic intervention to a cancer burdened organ or region of the body; they have the collective advantage of intensifying a treatment to a site of known disease while avoiding unnecessary systemic toxicity. They are most beneficial in circumstances where the tumor biology is favorable; that is, the site of regional metastatic spread is recognized to be the sole or life-limiting component of the patient’s disease. Two general categories of regional therapy include vascular infusion or perfusion and local ablation. Vascular isolation and perfusion of a cancer-bearing organ or region of the body using a recirculating extracorporeal perfusion circuit has been in clinical use for almost 50 years. Isolation perfusion was initially applied under normothermic conditions with chemotherapeutics alone and subsequently mild-to-moderate hyperthermia (38.5°C to 42°C) became a routine component of treatment when it was shown to be associated with improved response rates.1 In the early 1990s there was renewed interest in isolation. Most recently, there has been interest in the development of percutaneous techniques of isolated limb or liver infusion. The use of local ablative therapies such as radiofrequency ablation or irreversible electroporation has become increasingly utilized as a strategy to obliterate limited sites of disease in liver or other tissues. This chapter reviews the principles and techniques of isolation perfusion and infusion as well as the current status of these modalities in clinical practice. The various components of therapy that are routinely used are also reviewed.
There are advantages to isolation perfusion as a treatment technique in appropriately selected patients. Complete separation of the regional and systemic circulation can be achieved in most circumstances which allow for dose intensification of therapeutics to the cancer-burdened organ or region. The main parameter that limits dose escalation is the tolerance of the normal tissues within the perfusion field. In some circumstances, agents that cannot be administered systemically can be given via isolation perfusion. For example, tumor necrosis factor (TNF) is a biological agent that was demonstrated to have potent anti-tumor activity in murine models but had severe dose-limiting systemic toxicity in clinical trials when administered intravenously at doses far below the therapeutic threshold.2,3 However, when perfusate leak is controlled, TNF can be administered at doses that are manyfold higher than those tolerated systemically. It has been used in isolated limb, liver, lung, or kidney perfusion and is currently approved for use in Europe with melphalan administered via isolated limb perfusion (ILP) for patients with advanced extremity melanoma or sarcoma.4,5 There are data from experimental models demonstrating the feasibility of using isolation perfusion to selectively administer agents such as antisense oligonucleotides or recombinant mutant vaccinia virus within the perfusion field.6,7
Isolation perfusion is a surgical procedure performed under a general anesthetic with perfusion duration of 60 minutes for liver and 90 minutes for limb. The vascular supply of the cancer-burdened organ or region, such as liver or extremity, is isolated and all collateral blood flow to the area is controlled to avoid any leak of perfusate into the systemic circulation or leak of systemic blood into the perfusion circuit. Large cannulas are inserted into the feeding artery and draining vein and these are then connected to an extracorporeal bypass circuit consisting of a heat exchanger, reservoir, oxygenator, and roller pump. Mild-to-moderate hyperthermia is typically used by warming the perfusate to approximately 38.5°C to 40°C. Isolation perfusion allows one to deliver clinically significant levels of hyperthermia which has direct cytotoxic and synergistic anti-tumor effects with various chemotherapeutic and biological agents.8 Tissue temperatures must be monitored during treatment.
The parameters that must be monitored during perfusion include flow rates, line pressures, and reservoir volume. During ILP, continuous intraoperative leak monitoring is usually employed using either I-131 labeled human serum albumin or technetium-99-labeled erythrocytes. Complete vascular isolation is most typically achieved during isolation perfusion of the liver such that routine leak monitoring is no longer routinely used.9–11 Once the perfusion is completed, the vascular bed of the treated region is flushed with saline and colloid solution to remove any residual therapeutic agents. Finally, the native vascular blood flow is reestablished and therapy is completed. Because of the need to place indwelling vascular catheters during treatment, the patient must be systemically anticoagulated usually using heparin during perfusion. Activated clotting times (ACTs) above 350 seconds should be maintained throughout the perfusion. However, the anticoagulation effects can be effectively reversed with protamine sulfate and thawed fresh-frozen plasma.
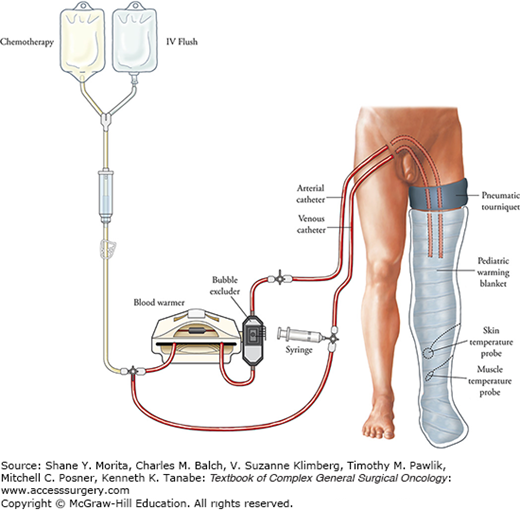
Isolated limb perfusion of the lower extremity is most commonly performed via the iliac approach where the external iliac vessels are exposed and cannulated; in the arm, the axillary vessels are used. Also, in the lower extremity ILP can be performed via the femoral or popliteal vessels and in the arm via the brachial vessels under appropriate clinical situations. For the approach to the iliac vessels, a lower abdominal “transplant” incision and a retroperitoneal approach is made. The external iliac artery and vein are dissected from their origin down to the inguinal ligament and small arterial branches and venous tributaries are ligated and divided. This is particularly important in the region of the inguinal ligament to prevent leak of perfusate into the systemic circulation. The hypogastric vein is ligated in situ and the hypogastric artery is temporarily occluded with a vascular occluding clamp. If possible, some of the branches of the hypogastric artery in the pelvis should be identified and ligated in order to prevent collateral flow across the pelvis. A Steimann pin is anchored into the anterior superior iliac spine and the external iliac vessels are cannulated with the catheter tips in each vessel positioned just below the inguinal ligament. An Esmarch tourniquet is snugly wrapped at the root of the extremity, held in place by the Steinmann pin that is anchored into the iliac wing, and the cannulas are connected to the extracorporeal bypass circuit (Fig. 10-1). If the foot is not afflicted with tumor then an Esmarch wrap can be applied tightly around the foot to prevent melphalan from perfusing the uninvolved tissues.
The extracorporeal perfusion circuit typically contains 1 L of perfusate which consists of 700 mL of a balanced salt solution, 1 U of type-matched packed red blood cells and 1500 U of heparin. The resultant hematocrit of approximately 25% provides adequate tissue oxygen retention, and perfusate containing higher hematocrits confer no additional benefit in preventing regional toxicity.12 Generally, flow rates in the range of 400–800 mL/min are achievable and adjusted depending upon line pressure, changes in reservoir volume, or the presence of a systemic perfusate leak based upon intraoperative monitoring. Once stable perfusion parameters and target tissue temperatures are obtained, melphalan at a dose of 10 mg/L limb volume for the lower extremity and 13 mg/L limb volume for the upper extremity are administered slowly into the venous reservoir and the perfusion extends for 90 minutes. Continuous intraoperative leak monitoring is not necessary during isolated hepatic perfusion (IHP) when a standard procedure for preparation of the liver is used; however, perfusate leak monitoring is used routinely as a component of ILP. Careful monitoring of leak can reduce the severity of systemic complications and may improve response rates.13 A gamma detection camera is positioned over the precordium of the heart for patients undergoing ILP, which serves as a stable reservoir of blood to measure radioactivity. Once the gamma detection camera has been positioned a small dose of radionuclide, usually technetium-99-labeled erythrocytes, is given systemically and baseline levels of radioactive counts are measured on a strip chart recorder. Then, a tenfold higher dose is administered into the perfusion circuit. Therefore, if a 10% leak of perfusate into the systemic circulation occurs, there will be a doubling of the amount of radioactivity compared to baseline. Leak rates using this system have been shown to correlate with measured leak rates with tumor necrosis factor or melphalan from the perfusate into the systemic circulation.14
Despite very careful preoperative preparation, during ILP the surgeon may encounter several situations which require adjustment in perfusion parameters to minimize a leak of perfusate or blood out of or into the perfusion circuit. Flow rates which indirectly affect arterial line pressure, reservoir volume, and leak of perfusate are continuously monitored. If there is leak of systemic blood into the perfusion circuit, this will be reflected by an increase in the reservoir volume in the circuit and can be remedied by increasing flow rates to increase line pressure, tightening the extremity tourniquet, or increasing venous pressure in the circuit by applying a partial occluding clamp on the venous outflow line. If there is a perfusate leak into the systemic circulation, this will be manifested by an increase in radioactive counts detected by the gamma camera and the strip chart recorder or one may see a decrease in reservoir volume in the perfusion circuit. Under these circumstances, one may decrease flow rates to lower the line pressure or tighten the tourniquet.15 Rarely, two-way leaks occur which is evidenced by changes in reservoir volume (generally a gain) as well as an increase in radioactivity on the strip chart recorder. This can be a particularly difficult and tricky situation to adequately control; typical steps would include decreasing flow rates to stop any systemic leak of perfusate, tightening the tourniquet and then placing the partial occluding clamp on the venous outflow line of the perfusion circuit. A leak rate of greater than 5% is encountered in only about 6% of ILPs and a leak rate of greater than 10% is encountered in only 1.4%.16
Isolated limb infusion (ILI) is conceptually similar to ILP. For the lower extremity, the patient is anticoagulated with 5000 to 10,000 units of heparin intravenously and the ACT is maintained above 350 seconds during the procedure. Two high-flow 6-Fr gauge catheters are inserted into the superficial femoral artery and vein of the uninvolved extremity. The tips of the catheters are positioned just above the knee joint of the afflicted limb using fluoroscopic guidance.17,18 A pneumatic tourniquet is placed around the proximal limb above the tips of the catheters (Fig. 10-2). If disease burden is high up in the limb, then an Esmarch tourniquet can be used. During ILI, isolation of the perfusate to the limb is entirely controlled by the effectiveness of the tourniquet. Temperature probes are then inserted into the skin and muscle and a warming blanket is placed around the limb. The catheters are then connected to an infusion circuit that includes a heat exchanger. Blood flow via ILI is generated by manually aspirating and delivering blood from and to the extremity via the cannulas using several 20 mL syringes that are in line with the circuit. The dose of melphalan administration is typically 7.5 mg/L limb volume for the lower extremity and 10 mg/L limb volume for the upper extremity. Dactinomycin is also used in the perfusate by some at a dose of 75 μg/L limb volume for the lower extremity and 100 μg/L limb volume for the upper extremity.
After the extremity is warmed to target temperature (37°C or greater), the chemotherapy is infused into the arterial line over 5 minutes and then circulated through the limb using the syringes to aspirate and infuse the perfusate for 30 minutes. This system does not use hyperthermia or oxygenation; much of the toxicity appears related to the combined effects of melphalan and hypoxia. Leak monitoring is not typically used. At the end of the infusion, the limb is flushed with 500 to 1000 mL of crystalloid, the tourniquet is released, and the catheters are removed.
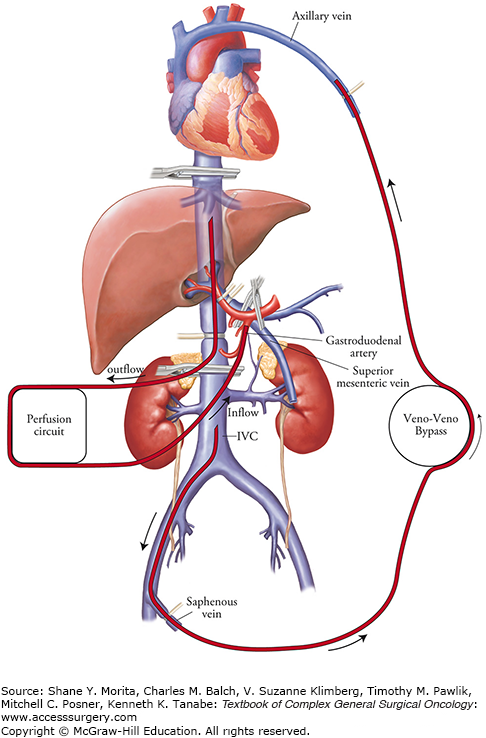
The unique vascular anatomy of the liver makes it an ideally suitable organ for isolated perfusion. Through a subcostal incision, the abdomen is evaluated; once it has been determined that there are no contraindications to proceeding with IHP, the liver is extensively mobilized. These includes division of the diaphragmatic attachments of the left and right hepatic lobes and complete dissection of the retrohepatic vena cava from the level of the renal veins to the diaphragm in order to prevent any leak of perfusate from the retrohepatic inferior vena cava. A cholecystectomy is performed and the porta hepatis structures are completely dissected and isolated. Cannulation for inflow to the liver is typically via the gastroduodenal artery; portal venous blood flow can be temporarily occluded without compromising cardiovascular stability during IHP.19 The venous effluent of the liver is collected from a cannula positioned in an isolated segment of the retrohepatic IVC and, therefore, during treatment the IVC flow must also be shunted (Fig. 10-3). The external veno-veno bypass circuit results in flow rates of approximately 1 L/min and stable cardiac parameters during treatment.20
FIGURE 10-3
Schematic illustration of the IHP perfusion circuit. The arterial inflow is via the gastroduodenal artery and venous outflow is collected from a cannula positioned in an isolated segment of retrohepatic vena cava. The inflow and outflow cannula are connected to a perfusion circuit. On the patient’s left is the veno-veno bypass circuit which shunts portal splanchnic and inferior vena cava blood flow back to the systemic circulation during therapy.
The perfusion circuit for the open IHP consists of a roller pump, membrane oxygenator, and a heat exchanger. The perfusate consists of 700 mL of a balanced salt solution and one unit of packed red blood cells (roughly 300 mL). In contrast to limb perfusion, arterial and venous blood gases must be monitored throughout the perfusion and usually between 20 and 40 mEq of NaHCO3– are administered in divided doses to maintain a perfusate pH between 7.2 and 7.3. The heat exchanger is utilized to warm the perfusate to maintain hepatic parenchymal temperatures between 38.5°C and 40°C. Uniform perfusion to both lobes of the liver can be observed by rapid and uniform increase in temperature in both lobes. The perfusion continues for 60 minutes and then the liver is flushed with 1500 mL of crystalloid followed by 1500 mL of colloid. The vascular structures are decannulated and repaired and normal liver perfusion is restored.
Percutaneous hepatic perfusion (PHP) has been under clinical evaluation as an alternative to IHP. PHP has the advantages of being a percutaneously administered method of regional hepatic perfusion; it is associated with less morbidity than IHP and can be repeated. PHP delivers high-dose chemotherapy to the liver via the proper hepatic artery; a double-ballooned catheter system (produced by Delcath Systems, New York) is positioned in the retrohepatic inferior vena cava to isolate and collect hepatic venous outflow. The venous effluent is then sent through an extra-corporeal filtration system using a centrifugal pump before being returned to the systemic circulation. The unique component of this system is a 16-Fr polyethylene double-ballooned catheter with one large lumen and three accessory lumina. A schematic of the PHP is shown in Fig. 10-4. The two low-pressure occlusion balloons are inflated independently using fluoroscopy. The cephalad balloon blocks the IVC superior to the hepatic veins, while the caudal balloon obstructs the IVC inferior to the hepatic veins, allowing complete isolation of hepatic venous outflow. The portion of the catheter between the two occlusion balloons consists of a fenestrated segment that feeds into the large, central lumen which exits the catheter from the proximal end. Hepatic venous flow is isolated using the occlusion balloon catheter, and blood from the central lumen is pumped through an extracorporeal circuit consisting of a centrifugal pump and two activated-carbon filter cartridges arranged in parallel. The filtered blood is returned to the systemic circulation via an internal jugular vein sheath (Fig. 10-4).
FIGURE 10-4
Schema of the PHP circuit using the Delcath Catheter System (Delcath, Norwalk, CT). Melphalan is infused over 30 minutes into an arterial catheter; hepatic venous effluent is collected by a double-ballooned catheter in the retrohepatic vena cava. Fluoroscopic image shows isolation of the hepatic veins.
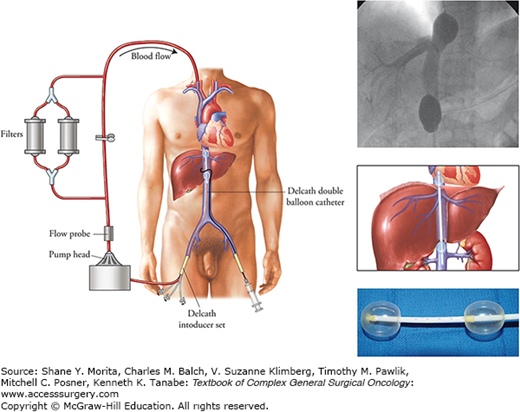
Treatments are administered with patients under general anesthesia. The hepatic arterial catheter is positioned percutaneously in the proper hepatic artery using fluoroscopy. In some cases, accessory arteries are embolized to ensure that the infused chemotherapy is administered solely to the liver. The double-ballooned catheter is inserted into the IVC percutaneously and positioned under fluoroscopic guidance. The catheter is attached to the extracorporeal circuit tubing and the outflow line of the filtration circuit is connected to the venous return catheter. Contrast medium is injected through the fenestrated portion of the catheter to ensure that the balloon catheter is properly placed and the hepatic venous outflow is isolated without leakage into the right atrium (Fig. 10-4). Once the system is confirmed to be functioning properly, melphalan, 1.5 mg/kg of ideal body weight diluted in 200 to 250 mL of saline, is administered as a 30-minute infusion via the hepatic artery. The extracorporeal filtration circuit continues for an additional 30 minutes after infusion.
Heparin is administered during the procedure to maintain the ACT at therapeutic levels. Protamine and fresh frozen plasma must be administered following the procedure to correct the coagulopathy and facilitate catheter and sheath removal. When the balloons are inflated, cardiopressors are temporarily required to maintain hemodynamic stability.
Isolated limb perfusion has been in clinical use for over 60 years; following the original report of normothermic ILP using chemotherapeutics in 1957 by Creech and Krementz,21 most investigators subsequently incorporated some degree of hyperthermia as closed circuit water-recirculating heat exchangers became available to replace the use of inefficient warm moist towels and infrared lamps to warm perfusate fluid. Stehlin reported results of ILP in 165 patients with extremity sarcoma or melanoma and observed that when the perfusate was warmed to 46°C and the average tissue temperature was 42°C, severe regional toxicity including pain, edema, blistering, and weakness was observed in 70% of patients.22 When the tissue temperature was reduced to 40°C or less, regional complications were minimal. Compared to historical controls treated identically at that institution, the addition of hyperthermia during ILP with melphalan in patients with extremity melanoma resulted in an increase in response rates from 35% to 80%.
The “modern” era of ILP really started about 20 years ago when the recombinant protein, tumor necrosis factor of TNF, was tested in regional perfusion. Because of the profound systemic toxicities associated with the protein, most centers performing ILP began to use very standardized operative approaches, specific and consistent patient selection criteria, routine leak monitoring, standard perfusion parameters including the degree of hyperthermia, dosing of melphalan, and perfusion interval, and routinely followed patients for response and survival. A representative summary of reports using ILP for patients with in-transit melanoma or sarcoma of the extremity is shown in Table 10-1.
Selected Series of Isolation Limb Perfusion or Infusion Using Chemotherapy and/or TNF
| Author | Trial Type | Agents | N | CR | PR | Comments |
|---|---|---|---|---|---|---|
| Lienard23 | Phase II ILP Melanoma/Sarcoma | Melphalan TNF 4 mg IFN | 29 | 90% | 10% | First report with TNF in ILP |
| Deroose29 | Database ILP Sarcoma | Melphalan TNF 1–4 mg | 208 | 18% | 53% | Limb salvage: 81% |
| 5-year OS: 42% | ||||||
| Bonvalot30 | Phase II ILP Sarcoma | Melphalan TNF 1 mg | 100 | 30% | 49% | Limb salvage: 87% |
| 3-year OS: 89% | ||||||
| DiFilipo31 | Database ILP Sarcoma | Doxorubicin TNF 1 mg | 75 | 34% | 48% | Limb salvage: 85% |
| 5-year OS: 62% | ||||||
| Hoven-Gondrie32 | Database ILP Sarcoma | Melphalan low- vs high-dose TNF | 98 | NR | NR | Limb salvage >65% |
| Low-dose TNF and shorter ILP efficacious | ||||||
| Grunhagen33 | Database ILP Melanoma | Melphalan low-dose TNF | 64 | 75% | Low-dose TNF efficacious | |
| Grunhagen36 | Database ILP Melanoma | Melphalan alone | 87 | 69% | 26% | Melphalan alone, good efficacy |
| Cornett35 | Phase RCT III ILP Melanoma | Melphalan +/− TNF | 58 | 25% | TNF not useful in ILP for melanoma | |
| 58 | 26% | |||||
| Alexander34 | Database ILP Melanoma | Melphalan +/− TNF | 91 | 69% | 26% | Median OS: 47 months |
| Median in-field PFS: 12 months | ||||||
| Brady40 | Phase II ILI Melanoma | Melphalan | 37 | 25% | 28% | Median duration response: 1 year (range: 5–32 months) |
| Raymond41 | Database ILI or ILP Melanoma | Melphalan | 188 | 55% (ILP) | Median duration response: 32 months | |
| 30% (ILI) | Median duration response: 24 months | |||||
| Wong18 | Database ILI Sarcoma/Melanoma | Melphalan | 104 | ORR sarcoma: 58% | ||
| ORR melanoma: 72% |
Lienard and Lejeune reported the first series of 29 patients treated with extremity melanoma or sarcoma using TNF and melphalan and demonstrated an unprecedented overall response rate of 100%.23 TNF is ideally suited for administration via isolation perfusion and is thought to exert its anti-tumor activity via effects on the tumor-associated neovasculature and augmenting the efficacy of chemotherapeutics administered with it. Current data indicate that TNF initially causes a rapid and selective increase in permeability in tumor neovasculature followed by stasis and obliteration of blood flow through the same capillaries.24–26 Interestingly, clinical data reporting use of TNF alone via isolation perfusion indicate that it has no meaningful independent anti-cancer effects.27,28
With respect to the use of ILP with TNF and melphalan for patients with unresectable extremity sarcoma, a number of reports have documented a limb salvage rate of greater than 80%29–31 (Fig. 10-5). It has been fairly well established that the original dose of 4 mg of TNF that was empirically derived may be unnecessarily high. Response and limb salvage rates appear comparable when a dose of 1 mg of TNF is used.30,31 Bonvalot30 and Deroose29 have reported limb salvage rates higher than 80% when ILP is used as a neoadjuvant therapy for patients with high-grade unresectable extremity sarcoma. DiFilipo31 showed similar results when doxorubicin was substituted for melphalan. Hoven-Gondrie and colleagues showed that a lower TNF dose (1 mg) and a shorter perfusion interval have comparable limb salvage and overall survival rates than ILP with higher doses (2 to 4 mg) and longer perfusion intervals.32 Currently, ILP with TNF and chemotherapy is approved for use in Europe for patients with high-grade unresectable extremity sarcoma.
FIGURE 10-5
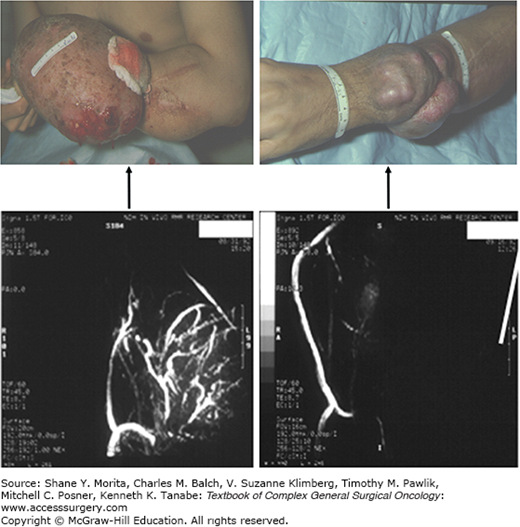
Pre- and postperfusion magnetic resonance angiograms (MRA) showing neovascularity in a large recurrent Ewing sarcoma arising on the dorsum of a forearm. The patient had small-volume pulmonary metastases and was treated with a palliative 90-minute hyperthermic ILP using TNF and melphalan. He had a significant regression (top panel) which lasted for 2 years until death from systemic disease progression. Three days posttherapy, complete obliteration of the tumor neovasculature was observed with no effect of perfusion on the native blood vessels in the extremity (bottom panel).
Several reports of TNF and melphalan administered via ILP for patients with in-transit extremity melanoma have reported complete response rates of greater than 70%33,34 but have failed to reproduce the initial 90% complete response rates reported by Lienard and Lejeune. The American College of Surgeons Oncology Group reported results of 133 patients with in-transit extremity melanoma randomized to one of two treatment arms: melphalan alone versus melphalan and TNF. The complete response rates at 3 months were 25% and 26% for the melphalan alone and the combined TNF and melphalan treatments, respectively.35 Although the trial showed no difference in outcomes; the complete response rates in both arms of that study were considerably lower than most other published series.34,36 For patients with moderate tumor burden from in-transit melanoma, the preponderance of current data indicates the following conclusions for ILP: adjuvant therapy as prophylaxis against in-transit disease is of no value, melphalan alone results in high response rates (complete response rates over 50%), and the addition of TNF does not improve efficacy or survival.37 However, a subset of patients with bulky in-transit melanoma metastases may benefit from the addition of TNF, even at low doses36 (Fig. 10-6). The use of alternate vasoactive agents such as histamine or IL-2 that are less toxic than TNF has been tested in experimental isolation perfusion models; both have synergistic anti-tumor activity with melphalan.38,39
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree