© Springer Japan KK 2017
Mototsugu Oya (ed.)Renal Cell Carcinoma10.1007/978-4-431-55531-5_1515. Refractory Mechanisms
(1)
Department of Urology, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan
Abstract
Vascular endothelial growth factor (VEGF)-targeted agents have brought significant progress in pharmacotherapy of advanced renal cell carcinoma (RCC). Some RCCs, however, appear to have innate resistance to treatment. Moreover, majority of patients who primarily respond to VEGF-targeted therapy eventually develop disease progression after prolonged treatment. The mechanism of resistance is supposed to be so multifactorial that it cannot be explained by single molecular events. Despite various factors including cytokines, bone marrow-derived cells, microenvironment, and genetic or epigenetic abnormality could be involved, tumor hypoxia seems to play one of crucial roles in most of the processes. The multiple mechanism in developing drug resistance might just represent the diversity of cellular response to hypoxia and nutrient deprivation, induced by the disruption of the blood vessels. In this chapter we comprehensively review current efforts aiming to clarify these process and to develop pharmacological strategies leading to overcome the drug resistance in RCC.
Keyword
Renal cell carcinomaAntiangiogenic therapyMechanisms of drug resistanceAcquired resistanceIntrinsic resistance15.1 Introduction
Several clinical studies have demonstrated that antiangiogenic therapies have a significant therapeutic benefit in terms of overall survival and disease control in patients with metastatic RCC [1–5]. The agents designed to blocking the angiogenic pathway include tyrosine kinase inhibitor (TKI), such as sorafenib, sunitinib, axitinib, and pazopanib, and a humanized anti-VEGF monoclonal antibody, bevacizumab. Despite these VEGF-targeted agents have brought significant progress in RCC pharmacotherapy, some RCCs appear to have innate resistance to treatment [6]. Clinical data of 4564 patients from 52 countries, treated with sunitinib for advanced or metastatic RCC, showed that 24 % of the patients were primarily refractory to the TKI, having progressive disease as best response or stable disease less than 3 months [7]. Through the histological examination on RCC specimens from patient receiving neoadjuvant TKI therapy, Tsuzuki et al. found TKI-induced vasculopathy of tumor vessels, which is characterized by decreased density of endothelial cells, within most of necrotic or degenerative area [8]. Interestingly, they also demonstrated that those tumors always accompanied TKI-insensitive areas which did not show vasculopathy, suggesting that TKI has different efficacies within same tumor. Intratumor heterogeneity could be one of the mechanisms explaining the limitation of this one-size-fits-all approach to systemic therapy for patients with kidney cancer [9].
Majority of patients who primarily respond to VEGF-targeted treatment eventually develop disease progression after prolonged treatment. This so-called acquired resistance has made it difficult to achieve complete and durable responses in patients with RCC and then eventually leads to death. Imaging studies have shown evidence that antiangiogenic therapy affects tumor perfusion in RCC [10, 11]. In patients with treatment resistance, those studies showed increasing rebound vascularization identified by contrast CT. These suggest that the mechanism that induced re-vascularization could be involved in the process of establishing acquired resistance.
In this chapter, we review the basic research data that inform the current view of the mechanism of resistance in antiangiogenic treatment. Those could provide insight into overcoming the clinical problem facing oncologists who care for patients with advanced RCC.
15.2 Mechanism of Resistance in Antiangiogenic Therapy
Resistance to anticancer drug therapy is defined as evidence of progressive disease measured by RECIST criteria despite continued treatment. Two general modes of tumor resistance have been proposed: intrinsic resistance which has no therapeutic benefit despite the administration of antiangiogenic drug and an adapted-acquired resistance, which induced progressive disease following a period of tumor control.
15.2.1 Intrinsic Resistance
Current evidence in literature have shown that under anti-VEGF therapy, particular clones can be selected that leads to development of more aggressive and metastatic tumor [12–14]. For example, mice bearing p53−/− human colorectal cancer cells have shown less response to anti-VEGF antibody therapy comparing to mice bearing p53+/+ tumors. Meanwhile, the selection of hypoxia-resistant p53−/− clones has been observed if the tumors from mixtures of p53+/+ and p53−/− clones are treated by VEGFR-2 inhibition [13]. However, it is hard to regard this scenario as likely in RCC, because p53 mutation is not a frequent event in RCC. Although mutation in a gene encoding a key tyrosine kinase receptor is a common cause of drug resistance development in other types of cancers [15, 16], it is not likely that this mechanism also could explain the drug resistance of anti-VEGF therapy. Because several evidences have demonstrated that resistance to VEGFR antagonists is reversible [17, 18], moreover, the target of VEGFR antagonists is endothelial cells, which are usually supposed to be less prone to mutation than genetically unstable tumor cells. However, several studies have been clarifying the genetic difference between tumor endothelial cells and their normal counterpart [19–21]. Bussolati et al. [20] obtained tumor-derived endothelial cells from renal cell carcinoma and observed that endothelial cells originated from tumor upregulated several proangiogenic growth factors and antiapoptotic survival pathway. Indeed, it has been demonstrated that tumor endothelial cells are cytogenetically abnormal and could have tumor-related mutation [22–24]. Possible mechanisms that result in tumor endothelial cell genetic mutation are not understood yet, but the involvement of tumor microenvironment producing cytokines leading to genetic instability and transdifferentiation of tumor cells into endothelial cells have been suggested [24]. Overall, several evidences have been suggesting the scenario that genetic mutation in tumor endothelial cells could be involved in the mechanism of resistance to antiangiogenic therapy; however, further comprehensive and meticulous analysis is warranted to prove this hypothesis.
15.2.2 Acquired Resistance
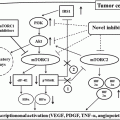
In orthotopic tumor models of glioblastoma in which VEGF or HIF1α was genetically or therapeutically blocked [25–27], hypoxia in the tumor microenvironment accompanied with initial tumor shrinkage could eventually lead or facilitate increased invasiveness along host vessels or recurrent tumor growth. Increased invasiveness and metastasis in mice also have been observed in pancreatic neuroendocrine carcinoma, exhibiting marked hypoxia following inhibition of VEGFR and PDGFR signaling by sunitinib [28]. Tumor-independent (host-mediated) pathways of resistance to angiogenesis inhibition have also been demonstrated to facilitate metastasis [29]. Although several evidences have not been definitive yet, there are six distinct different mechanisms involved in acquired resistance to antiangiogenic therapy. Those mechanisms are (1) mediated by the production of pro-angiogenic factors, (2) recruitment of pericyte coverage, (3) recruitment of bone marrow-derived cells, (4) epigenetic change to adapt environment, (5) lysosomal sequestration, and (6) increasing invasiveness and metastasis through epithelial to mesenchymal transition (EMT).
15.2.2.1 Upregulation of Pro-angiogenic Pathway
HGF
Ligand-dependent c-MET activation through paracrine HGF induces proliferation, survival, and invasiveness of solid tumor through a series of signaling pathways including PIK3/Akt, STAT, and Ras/Mek. It also regulates tumor angiogenesis by inducing endothelial cell migration and tubular formation. The significant role of the HGF/c-met pathway in the mechanism of resistance to anti-VEGFR therapy has been indicated by several authors [30, 31]. Shojaei et al. [32] demonstrated that HGF levels in the sunitinib-resistant xenograft tumor such as mouse lymphoma (EL4) and Lewis lung carcinoma (LLC) are much higher when compared to that of sunitinib-sensitive tumor such as B16F1 mouse melanoma and TIB6 mouse myeloma cells, especially after treated by sunitinib. Combination of sunitinib and selective c-MET inhibitor had an additive effect of inhibiting the growth of sunitinib-resistant xenograft. Moreover, treatment efficacy of sunitinib for B16F1 or TIB6 cells was significantly decreased after the exposure of tumor cells to HGF. This approach clearly confirms the significance of c-MET activation in the mechanism of developing anti-VEGFR drug resistance in xenograft of several malignancies. Recently, cabozantinib, which is a novel tyrosine kinases inhibitor of c-MET and VEGFR2, has been highlighted for targeting both axes, expected to offer significant benefit comparing to targeting each individual pathway [33]. In phase I trial for RCC, the patients, most of whom had VEGF pathway inhibiting therapy, have shown a favorable safety profile and antitumor activity [34].
FGF
Casanova et al. provided the first report of fibroblast growth factor (FGF) involvement in the resistance to anti-VEGFR therapy by using RIP-Tag2 model, which is a genetically engineered mouse model producing pancreatic islet carcinoma [35]. In their study, although VEGFR2 blockade with an antibody initially achieved an impaired tumor growth and angiogenesis, significant regrowth of the treated tumor occurred during 4 weeks of treatment. Those tumors which grew during anti-VEGFR2 therapy showed a more invasive and malignant phenotype. Quantitative mRNA expression analysis in relapsing tumors revealed a significant upregulation of a set of proangiogenic factors, such as FGFs, ephrins, and angiopoietin, when compared with untreated tumors. In the condition of hypoxia, upregulation of some of these proangiogenic families was also observed in the cultured cell line, derived from RIP-Tag2 tumors. Interestingly, blocking of FGF by using a soluble form of the FGF receptor achieved a remarkable decrease in tumor volume and angiogenesis during the regrowth phase, especially when they are combined with anti-VEGFR2 treatment. Those indicated that increased production of FGFs could be involved in alternative pro-angiogenic pathway and partly explained the mechanism of development of resistance to VEGFR blockade. Subsequent related studies also have provided the effect of FGF blockade in the face of VEGF inhibition. Welti et al. reported that although sunitinib alone could provide a significant inhibition of endothelial tube formation in the presence of VEGF [36], FGF2 could suppress this antiangiogenic activity of sunitinib. Addition of FGF2 to cultures incubated with VEGF and sunitinib increased the endothelial proliferation and de novo tubule formation. Furthermore, Bello et al. demonstrated by using a human RCC xenograft model that renal tumors that had developed resistance to sunitinib still had sensitivity to a dual inhibitor of VEGFR and FGFR tyrosine kinase, E-3810 [37], suggesting the clinical utility of FGF antagonist in advanced RCC. However, the recent clinical trial using dovitinib, a multi-tyrosine kinase inhibitor of FGFR, VEGFR, and PDGFR, could not prove the superiority of FGFR-targeting therapy in the treatment of anti-VEGFR-refractory RCC patients [38].
IL-8
Many of the proangiogenic factors upregulated under the hypoxic microenvironment of tumor contain hypoxia-response element (HRE) on their gene promoter [39]. This suggests that the HIF pathway partly regulates the compensatory gene expression in the tumors which has responded to antiangiogenic treatment, leading to the development of intratumoral hypoxia. However, Mizukami et al. revealed that even in the absence of functional HIF-1 protein, IL-8 could be induced and maintain vascularity of colon cancer xenograft. They also confirmed that neutralizing antibody to IL-8 achieves the regression of xenograft tumor lacking HIF-1 [40]. Wyscozynski et al. showed that blockade of both MAPK and PI3K-AKT pathways was necessary to inhibit the hypoxia-induced IL-8 expression in rhabdomyosarcoma cells, suggesting that nuclear factor (NF)-KB and activating protein (AP)-1 have an essential role in regulating its gene expression [41].
The potential role of IL-8 on mediating resistance to sunitinib treatment in RCC was demonstrated by Huang et al. [42]. They established a sunitinib-resistant human RCC xenograft model by administrating minimal dosage of drug with an intermittent dosing schedule. The levels of various secreted human cytokines derived from the xenograft tumor were screened. They found that IL-8 level of plasma sample from mice bearing sunitinib-resistant tumor was higher than that from mice with sunitinib-sensitive tumor. To demonstrate the functional significance of IL-8 in development of sunitinib resistance, neutralizing antibody to IL-8 was used in xenograft models. The combination treatment with IL-8 neutralizing antibody and sunitinib had a significant effect in decreasing the size and microvascular density of sunitinib-refractory tumor, even though the neutralizing antibody alone did not reduce the growth of the tumor. They conclude that inhibition of IL-8 function could resensitize the resistance tumors to sunitinib treatment, which result in decreasing RCC growth.
PlGF
Although placental growth factor (PlGF) is a member of the VEGF family, it has a different characteristic from that of VEGF-A isoforms, which bind to VEGFR-1 and VEGFR-2. There is much evidence that VEGFR-2 is the major mediator of VEGF signaling pathway in endothelial cells [43], but PlGF exclusively binds to VEGFR-1, which is expressed by tumor cells, endothelial cells, bone marrow-derived proangiogenic and proinflammatory cells, and stromal cells [44]. The functional properties of VEGFR-1 are still under debate, because they seem to be different depending on the cell type. Although VEGFR-1 has ten times higher affinity to VEGF than VEGFR-2, it undergoes weak tyrosine phosphorylation in response to VEGF [43]. Therefore, VEGFR-1 is considered rather as a decoy receptor, which is able to negatively regulate the activation of VEGFR-2 by VEGF. Indeed, PlGF-deficient mice are healthy and fertile under physiological condition. However, in pathological conditions such as wound healing and inflammation, defect in pathological angiogenesis becomes obvious when a PlGF-deficient mouse was analyzed [45, 46]. The therapeutic potential of PlGF blocking in tumor growth was initially demonstrated by Luttun et al. [47]. Later on, Fisher et al. [48] demonstrated in a xenograft model of melanoma and colonic and pancreatic cancer that blockade of PlGF with a monoclonal antibody enhanced the antitumor activity of anti-VEGFR-2 therapy by inhibiting tumor angiogenesis and intratumoral macrophage recruitment. As for RCC, Rini et al. have revealed that serum levels of PlGF are increased in patients treated with anti-VEGFR therapy [49]; however, the treatment efficacy of PlGF blocking strategy in anti-VEGF treatment-resistance RCC is still under research [50].
Angiopoietin
In addition to the VEGF-VEGFR system, Tie receptors, and their ligands, angiopoietin has been regarded as second tyrosine kinase receptor signaling system which is specific to vascular endothelial cells [51]. This signaling is supposed to have an essential role in maturation and stabilization of the blood vessels. In RIP-Tag2 model, upregulation of angiopoietin mRNA was demonstrated in the tumor relapsing after initial anti-VEGFR2 treatment [35]. The association between evasive tumor resistance and anti-VEGF therapy and adaptive upregulation of Ang2 was revealed in the same model recently by Rigamonti et al. [52]. Therefore, it is not surprising that several recent evidences have shown that angiopoietin production decreases the effect of VEGFR-targeted therapies in other types of cancer. For example, Hashizume et al. demonstrated that the combination of Ang2 inhibitor with anti-VEGF antibody could limit the tumor growth and vasculature expansion more effectively than either of those agents alone in a colon cancer xenograft model [53]. The efficacy of human anti-Ang2 monoclonal antibody was confirmed in several other types of xenograft tumor, including RCC [54]. However, as for RCC, there is only limited evidence which suggests the therapeutic benefit of the combination therapy targeting Ang2 along with VEGF inhibition [55].
15.2.2.2 Recruitment of Pericyte Coverage
Pericytes are perivascular support cells, which are not only providing a scaffold but communicate with endothelial cells by direct physical contact and reciprocal paracrine signaling. Tumor pericytes appear to be responsible for maintaining the integrity and functionality of the blood vessels [56], even though they are more loosely attached to tumor vessels and less abundant in the tumor tissue, when compared to healthy tissue [57]. Pericytes may play a role as a local source of VEGF for adjacent endothelial cells, and several studies have indicated that pericytes protect the vascular network against anti-VEGF treatment. For example, Helfrich et al. [58] demonstrated that tumor vessels in human melanoma metastasis, which grew during bevacizumab therapy, were featured by increase in the diameter of blood vessels and normalization of the tumor vascular bed covered by mature pericytes, compared with those from patients without anti-VEGF treatment. The blood vessels of treatment-resistant tumor had also an enhanced expression of desmin and α-SMA, which are immature and mature markers of pericytes, respectively, suggesting that mural cell differentiation and stabilization of the vascular wall could contribute to the therapeutic effect of antiangiogenic therapy. Indeed, several approaches have done targeting tumor pericytes to overcome the resistance to VEGF pathway inhibition. Tyrosine kinase inhibitor of PDGF receptor has been considered as a relatively selective pericyte-targeting drug, which could disrupt pericyte support [59]. Pietras et al. revealed that PDGF inhibition could increase sensitization of endothelial cells to antiangiogenic chemotherapy, resulting in regression of tumor vasculature in a xenograft model of pancreatic islet cancer [60]. However, inhibition of PDGF might be a double-edged sword, since the decreased coverage by pericytes could lead to vascular destabilization and subsequent escape of tumor cells into the blood vessel, resulting in hematogenous metastasis [61]. To date, clinical trial for renal cell carcinoma treated by the inhibition of VEGF and PDGF pathway showed no treatment benefit compared to single anti-VEGF therapy, despite the fact that the combined regimen exhibited increased toxicity [62].
15.2.2.3 Recruitment of Bone Marrow-Derived Cells
Hypoxia induced by tumor vessel regression through anti-VEGF treatment recruits not only other proangiogenic factors but also bone marrow-derived cells, which facilitate vascular remodeling and tumor growth. Those cells are tumor-associated macrophages [63], immature TIE2+ monocytes [64], VEGFR1+ hemangiocytes [65], and CD11b+ myeloid-derived suppressor cell [66, 67], all of which expressing CXCR4 receptor. Ceradini et al. showed marked increase in SDF-1 mRNA and protein expression in ischemic tissue which was proportional to the reduction of oxygen tension in the tissue [68]. SDF-1 expression was induced by HIF-1, then CXCR-4 positive circulating cells were recruited to the site of ischemic tissue. Du et al. [69] had shown in mouse model of glioblastoma that HIF1 arufa induces recruitment of bone marrow-derived CD45+ myeloid cells, through the upregulation of VEGF and SDF-1 in tumor cell. Those monocytic cells comprised of heterogeneous population, such as CD11b+ monocyte cells, F4/80+ macrophages, TIE2-expressing monocytes, and VEGFR1 hemagiocytes. Shaked et al. illustrated that antiangiogenic treatment leads to acute reactive mobilization of circulating endothelial progenitors to damaged vessels, which could contribute to the rapid regrowth of xenograft tumor [70]. As for RCC, loss of VHL function has been shown to overexpress both CXCR4 and SDF-1 through the constitutive activation of HIF-1 protein [71]. Pan et al. indicated that the expression of CXCR4 was associated with metastatic behavior in RCC xenograft mice and neutralization of SDF-1 could reduce metastasis in this model [72]. Recently, increased tumor infiltration of CD11b+ myeloid cells, comparing to untreated control, was demonstrated in RCC xenograft treated by sunitinib [73]. Those preliminary evidences suggest CD11b+ myeloid might play a substantial role in acquired resistance to antiangiogenic treatment in RCC.
15.2.2.4 Epigenetic Change to Adapt Environment
Recent studies have focused on the epigenetic change of gene expression in cancer under hypoxic environment [74]. Mechanism of epigenetic regulation involves DNA methylation, histone modification, and nucleosome remodeling, all of which control gene expression without alternation of DNA sequences. High-throughput genetic studies of RCC have identified the several mutated genes whose functions are implicated in epigenetic modification, such as PBRM1, UTX, SETD2, and JARID1C [75–77]. However, mutation patterns of those genes were not concordant between primary and metastatic region, and mutation of SETD2 histone methyltransferase and JARID1C histone demethylase genes tend to be identified in metastatic sites [78]. Histones are regulators of chromosomal activity by altering electrostatic charge by changing chromatic structure or by providing protein recognition sites through specific modification. Histone modifications included methylation, acetylation, and ubiquitination. In active gene, promoters are mainly marked by methylated H3 at lysine 4 (H3K4me3), and transcribed regions are enriched for H3K36me3 and H3K79me2. Histone H3K9 methylation and H3K27 tri-methylation usually associate with gene repression [79]. Dysregulation of histone-modifying enzymes in RCC leads to global change in those histone modifications [80–82], which may contribute to aberrant gene expression.
Screening of genes regulated by hypoxia has identified promoters of several histone demethylase (JMJD1A, JMJD2B, and JARID1B) as direct binding target of HIFs [83–85].
Krieg et al. [86] demonstrated that the subset of protumorigenic genes, such as adrenomodullin (ADM) and growth and differentiation factor 15 (GDF15), induced by hypoxia, are regulated by JMJD1A-dependent histone modification. In their study, loss of JMJD1A did not have an effect on growth of cancer cells in vitro but reduced the rate of xenograft tumor growth. Those suggest that induction of JMJD1A in the cells exposed to a hypoxic environment facilitates both hypoxic and oncogenic gene expressions and then enhances tumor growth.
Recent study has elucidated that JMJD1A inhibition reduces vascular formation and macrophage infiltration into xenograft tumor tissue [87]. Moreover, JMJD1A inhibition increases the effect of anti-VEGF therapy possibly through the reduction in the expression of tumor-derived FGF2, HGF, and Ang2. These data indicate that targeting epigenetic modifier, such as JMJD1A, could be a novel strategy to overcome the resistance to antiangiogenic therapy.
15.2.2.5 Lysosomal Sequestration
Gotink et al. [88] have revealed that prolonged exposure of RCC cells to sunitinib in vitro resulted in transient resistance to the drug whose mechanism could be explained by not decreased but increased drug concentration within the cell. Their imaging analysis revealed that sunitinib was predominantly colocalized with lysosomal staining. Those subcellular localization of sunitinib was disturbed by co-incubation with bafilomycin A1, which abolished the acidification of lysosomes. Sunitinib has chemical features of a hydrophobic, weak base; therefore, it is supposed to be easily sequestered in acidic lysosomes. Although sequestering sunitinib from a cytoplasmic compartment was associated with decreasing growth inhibitory activity, phosphorylation levels of ERK and Akt were similar between parental and sunitinib-resistant cells. Those suggest that increased lysosomal sequestration could be a novel possible mechanism of resistance to sunitinib.
15.2.2.6 EMT
Epithelial to mesenchymal transition (EMT) is a recent recognized phenomenon. During EMT, epithelial cells lose cell-cell adhesion and begin to show the migratory and invasive phenotype. Molecular features of EMT are characterized by loss of epithelial markers such as E-cadherin or by upregulation of mesenchymal markers such as fibronectin and vimentin. In advanced RCC, parts of the tumor commonly show a sarcomatoid pattern, and transition of epithelial clear cells to sarcomatoid ones is considered as EMT in RCC [89]. Several EMT regulators play a critical role as E-cadherin repressor. Among them, Snail appears to be an important player in development of sarcomatoid RCC progression [90].
Several evidences have supported the idea that EMT has associated with the mechanism of drug resistance including gefitinib and paclitaxel [91, 92]. To explore the relation of EMT with a resistance to antiangiogenic treatment, Hammers et al. [17] obtained skin metastatic lesion from a patient with advanced clear cell RCC, which initially had responded to sunitinib treatment but eventually developed resistance. The minced metastatic tumor tissue was implanted subcutaneously in a nude mouse, and then sunitinib treatment was performed on the fourth and fifth generation of primary xenograft. Surprisingly, the tumor regained sensitivity to the same sunitinib treatment, coupled with the reduction in microvascular density. Moreover, although the primary skin metastatic tissue showed pure sarcomatoid feature without clear cell pattern, conventional clear cell histology was restored during the development of xenograft tumor. At the same time, mesenchymal markers, such as vimentin and HIF-1, were upregulated in the spindle-shaped cells of sarcomatoid structure. These suggest that an EMT-like phenotype in a patient with clear cell RCC associated with acquired resistance to antiangiogenic treatment.
Several studies have suggested the link between the EMT and the expression of cancer stem cell (CSC) phenotype [93, 94]. CD44 is one of putative CSC surface markers in several types of cancer. Breast cancer cells with CD44 expression are resistant to chemotherapy [95]. In fact, chemotherapy itself could lead to increase the number of cells with CD44 high expression, which suggests that drug-induced CSC phenotype may play a crucial role in the mechanism of acquired drug resistance [96]. Mikami et al. demonstrated that TNF-α stimulation on ccRCC cells enhanced not only EMT marker but upregulated the expression of CD44. [97] Interestingly, hypoxic condition also could induce both CD44 and TNF-α expression. They also demonstrated that the expression of TNF-α and CD44 was predominant in high-grade ccRCC, and those had an inverse correlation with progression-free survival of the patient. Moreover, CD44 was highly expressed in metastatic ccRCC specimens which had been resected from patients receiving sunitinib treatment. Those data indicated that CD44 induced by TNF-α under specific microenvironment, such as intratumoral hypoxia, could be involved in the process of acquired antiangiogenic drug resistance in ccRCC.
15.3 Conclusion
The mechanisms of nonresponsiveness to VEGF-targeted therapies, we have outlined above, clearly show that the resistance to antiangiogenic treatment is so multifactorial that it cannot be explained by single molecular mechanism. Despite various factors including cytokines, bone marrow-derived cells, microenvironment, and genetic or epigenetic abnormality could be involved, tumor hypoxia seems to play a crucial role in most of the processes. In other words, the multiple mechanism in developing drug resistance just represents the diversity of cellular response to hypoxia and nutrient deprivation, induced by the disruption of the blood vessels. Interestingly, there may be a delicate balance between destruction of vasculature and induction of intratumoral hypoxia, because tumor vascular regression possibly leads to transient vessel normalization by reopening of previously collapsed vessels, which could facilitate tumor growth and metastasis [98, 99]. Furthermore, deprivation of oxygen and nutrient supply not only decreases tumor growth but selects or increases the malignant phenotype of cancer cells. Those complexities have undermined the possibility that the inhibition of just one additional proangiogenic cytokine would consistently solve the problem of resistance. Targeting various stress-induced pathways activated by hypoxia seems to be an attractive strategy to overcome the drug resistance in RCC.
The problem of permanent intrinsic resistance to molecular-targeted therapy is usually supposed to be resolved through the comprehensive analysis of individual genetic patterns. However, different from other types of solid tumor, such as breast and lung cancer, biomarkers, which could easily predict treatment outcome at the onset, have not been identified in RCC yet. The presence of complex genomic abnormality within a different region of a tumor, and a different metastatic site, could be the reason for the difficulty in RCC [9]. Tissue collection protocols based on multi-region tumor analyses to identify relatively common genetic change should be served in clinical trials to find more accurate predictors of disease biology and treatment outcome.