Highlights
- •
Over 2,000 cases were studied for infection prevention in TRUS-PBx.
- •
Cefmetazole (CMZ)+levofloxacin (LVFX) reduced febrile UTI incidence to 0.20%.
- •
CMZ+LVFX also reduced medical costs compared to cefazolin+LVFX.
Abstract
Introduction
Fluoroquinolones (FQ) are currently the first choice as prophylactics for transrectal ultrasound-guided prostate biopsy (TRUS-PBx). However, infections caused by FQ-resistant or extended-spectrum β-lactamase producing Escherichia coli remain a significant concern. Although cefmetazole (CMZ) is effective against these resistant E. coli strains, there are only a few reports on its use in TRUS-PBx. We investigated the efficacy of antimicrobial prophylaxis (AP) for TRUS-PBx using intravenous CMZ and oral levofloxacin (LVFX).
Methods
This single-center retrospective observational before-and-after study was conducted between January 2014 and December 2023 at Komaki City Hospital, Japan. The incidence of febrile urinary tract infection (UTI), urosepsis, bacteremia, readmission, abscess, and healthcare-related costs after TRUS-PBx were compared between individuals who received a single dose of intravenous cefazolin (CEZ) and oral LVFX and those who received single doses of intravenous CMZ and oral LVFX. The risk factors for post-TRUS-PBx febrile UTI were analyzed using multivariable logistic analysis.
Results
The incidence of febrile UTI after TRUS-PBx was 0.77% (9/1,168) in the CEZ+LVFX group and 0.20% (2/1,008) in the CMZ+LVFX group. Complications such as urosepsis (5 cases), bacteremia (3 cases), abscess (2 cases), and readmission (3 cases) were observed only in the CEZ+LVFX group. Multivariable analysis indicated that the use of CMZ+LVFX significantly decreased febrile UTI after TRUS-PBx (odds ratio: 0.20, 95% confidence interval: 0.04–0.98, P = 0.047). CMZ+LVFX use reduced healthcare-related costs by JPY 975.5 (USD 6.8) per TRUS-PBx compared to CEZ+LVFX.
Conclusions
Empirical AP with CMZ+LVFX before TRUS-PBx reduced the incidence of infectious complications and healthcare-related costs.
1
Introduction
Transrectal ultrasound-guided prostate biopsy (TRUS-PBx) is the standard procedure for prostate cancer diagnosis worldwide. Urinary tract infections (UTIs) remain one of the most serious complications related to TRUS-PBx. The percentage of symptomatic genitourinary tract infections following prostate biopsy ranges from 0–6.3% [ ]. The incidence of infectious complications (ICs) associated with TRUS-PBx has increased over the years, thereby increasing healthcare costs [ , ].
Antimicrobial prophylaxis (AP) plays an important role in preventing ICs after TRUS-PBx. Fluoroquinolones (FQ) and cephalosporins are widely used as standard AP [ ]. Despite the appropriate use of AP before TRUS-PBx, the rising incidence of ICs owing to FQ-resistant and extended-spectrum β-lactamase (ESBL)-producing bacteria has emerged as a serious issue [ ].
Cefmetazole (CMZ) is a second-generation cephalosporin that is classified within the cephamycin subgroup. CMZ is stable against hydrolysis by ESBL and has a relatively narrow spectrum of gram-negative rods compared to carbapenems. Escherichia coli is the primary causative organism in infections following prostate biopsy [ ]. In Japan, nearly all E. coli strains are susceptible to CMZ [ ]. Notably, the intravenous (IV) administration of 2 g of CMZ, 30 minutes to 5 hours before TRUS-PBx, results in a prostate tissue concentration of 17.8 ± 9.7% relative to the blood concentration [ ]. However, it remains unclear whether the combination of CMZ and levofloxacin (LVFX) is more effective than other regimens in preventing ICs of TRUS-PBx. Therefore, this study aimed to investigate the incidence of ICs and the cost-effectiveness of CMZ+LVFX as an empirical AP for TRUS-PBx.
2
Patients and methods
2.1
Study design, population, and definitions
In this retrospective observational before-and-after study, we reviewed the medical records of patients who underwent TRUS-PBx between January 2014 and December 2023 at the Komaki City Hospital (558 beds until April 2018 and 520 beds since May 2019), a tertiary care center in Aichi Prefecture, Japan.
AP administration during TRUS-PBx was divided into 3 periods. The route of administration and dosage of the antimicrobials were as follows: cefazolin (CEZ) (IV), 1 g; CMZ (IV), 1 g; and LVFX (oral), 500 mg. From January 2014 to July 2017, the AP consisted of a single dose (SD) of CEZ before TRUS-PBx and multiple doses (MD, 5 days) of LVFX after TRUS-PBx. From August 2017 to April 2019, CEZ and LVFX SDs were administered as AP before TRUS-PBx. Starting from May 2019, SDs of CMZ and LVFX were administered as AP before TRUS-PBx. The exclusion criteria were as follows: age < 18 years, untraceable cases, use of alternative antibiotics, use of antibiotics for infections other than those related to TRUS-PBx within 14 days after TRUS-PBx, use of antibiotics within 14 days before TRUS-PBx, and data loss.
All TRUS-PBx procedures included overnight hospitalization for observing complications. Patients without any complications were discharged the following morning. All biopsies were performed after rectal cleansing using a single glycerin enema and intrarectal disinfection using povidone-iodine.
The following data were collected from electronic medical records: age, serum prostate-specific antigen, diagnosis of prostate cancer, prostate size, obesity (body mass index [BMI] ≥ 25), history of antibiotic use within 6 months, hospitalization within last 3 months, history of prostatitis, and history of prostate biopsy. The following data were collected as risk factors: the presence of an indwelling urethral catheter, dysuria (residual urinary volume ≥ 100 ml), prostate volume ≥ 75 ml, poorly controlled diabetes mellitus (HbA1c ≥ 8.0%), and corticosteroid use [ ].
The primary outcome was the incidence of febrile UTI, defined as a diagnosed UTI with a fever of 37.8°C or higher within 14 days after TRUS-PBx. Secondary outcomes included incidences of urosepsis, bacteremia, abscess, and readmission. Urosepsis was diagnosed if the symptoms met two or more of the following criteria: (1) a temperature > 38°C or < 36°C, (2) heart rate > 90/min, (3) respiratory rate > 20/min or PaCO 2 < 32 mmHg, and (4) a peripheral blood leukocyte count > 12,000/mm 3 or < 4,000/mm 3 [ ].
2.2
Comparison of costs
The costs associated with TRUS-PBx and subsequent ICs were based on a cost-effectiveness model [ ]. In this model, we estimated healthcare-related costs based on the total cost of AP for TRUS-PBx, the cost per febrile UTI, and the incidence of febrile UTI after TRUS-PBx. The cost of AP was calculated based on the prevailing drug prices in Japan as of December 2023. The cost of each IV antibiotic agent was calculated after adding the cost of the two-chamber formulation saline. The cost of each drug (in Japanese Yen, JPY) was as follows: LVFX, JPY 77.4; CMZ, JPY 441.0; CEZ, JPY 291.0; and two-chamber saline formulation, JPY 204.0. The cost per febrile UTI indicates the average cost of additional hospitalization and outpatient medical expenses incurred owing to ICs per patient. Healthcare-related costs per patient were estimated using the following formula: cost of total prophylactic antibiotics (including the two-chamber formulation of saline) + incidence of febrile UTI × average cost per febrile UTI. A conversion rate of 1 US dollar (USD) to 144 JPY was used as of December 2023.
2.3
Statistical analyses
Categorical variables were expressed as totals and percentages and analyzed using Fisher’s exact test, whereas continuous variables were expressed as means and interquartile ranges (IQR) and analyzed using the Mann–Whitney U test. We performed a stratified analysis to evaluate the relationship between risk factors and the incidence of febrile UTI by comparing patients with and without risk factors using Fisher’s exact test.
For the primary analysis, we used multivariable logistic regression to assess whether using CMZ+LVFX as a prophylactic reduced the incidence of febrile UTI. Adjusted variables were first selected based on their clinical relevance and then further refined using statistical methods [ ]. From a clinical perspective, the following adjusted variables were considered: age ≥ 73, number of biopsy cores > 12, obesity (BMI ≥ 25), history of antibiotic use within 6 months, hospitalization within last 3 months, history of prostatitis, history of prostate biopsy, indwelling urethral catheter, prostate volume ≥ 75 ml, dysuria (residual urinary volume ≥ 100 ml), diabetes mellitus (HbA1c ≥ 8.0%), and corticosteroid use. Using statistical methods, we selected the main model as the one with the lowest Akaike Information Criterion, with CMZ+LVFX designated as the prophylactic and included in the model as the primary explanatory variable.
A significance level of 5% was used for all analyses. All statistical analyses were performed using EZR, which is based on R (version 4.3.1; R Foundation for Statistical Computing, Vienna, Austria) [ ].
2.4
Ethics statement
This study adhered to the Japanese ethical guidelines for epidemiological studies, and all study protocols were approved by the Institutional Review Board of Komaki City Hospital (Approval No. 231048). The requirement for written informed consent was waived owing to the retrospective nature of the study.
3
Results
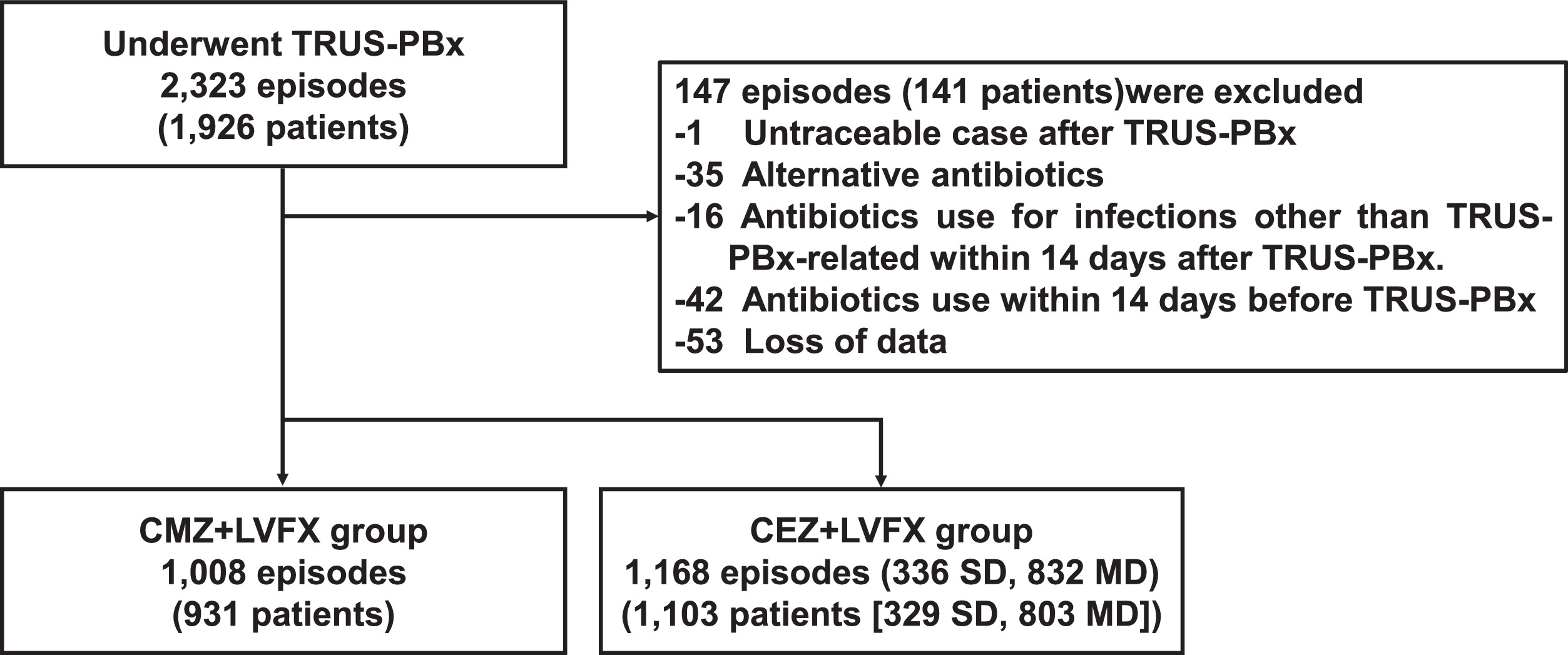
Fig. 1 illustrates the flowchart outlining the patient inclusion process. The number of episodes requiring CEZ+LVFX-MD, CEZ+LVFX-SD, and CMZ+LVFX administration was 832, 336, and 1,008, respectively. The characteristics of patients are summarized in Table 1 . Patients in the CMZ+LVFX group were significantly older than those in the CEZ+LVFX group (73 [IQR: 68–78] vs.70 [IQR: 65–74], P < 0.001). The patients in the CMZ+LVFX group were more likely to have a history of antibiotic use within 6 months (7.94% vs. 5.48%, P = 0.024), especially fluoroquinolones. The percentage of patients with at least 1 risk factor was nearly identical.
| Characteristics | CEZ+LVFX | CMZ+LVFX | P -value |
|---|---|---|---|
| n = 1,168 | n = 1,008 | ||
| General information | |||
| Age (year) | 70 (65–74) | 73 (68–78) | < 0.001 |
| PSA (ng/ml) | 7.66 (5.49–12.69) | 8.50 (5.61–14.28) | 0.007 |
| Prostate volume (ml) | 31.38 (23.43–46.20) | 32.28 (23.33–46.72) | 0.767 |
| Number of biopsy cores | 12 (12–12) | 12 (12–12) | 0.006 |
| Diagnosis of prostate cancer | 525 (44.9) | 488 (48.4) | 0.111 |
| Duration of LVFX administration | |||
| Single dose | 336 (28.8) | 1,008 (100) | |
| Multiple dose | 832 (71.2) | 0 (0) | |
| Obesity (BMI ≥ 25) | 207 (17.7) | 185 (18.4) | 0.737 |
| History of antibiotic use within 6 months | 64 (5.48) | 80 (7.94) | 0.024 |
| Fluoroquinolone | 26 (2.23) | 51 (5.06) | |
| Hospitalization within last 3 months | 9 (0.77) | 14 (1.39) | 0.207 |
| History of prostatitis | 19 (1.63) | 15 (1.49) | 0.863 |
| History of prostate biopsy | 199 (17.0) | 195 (19.3) | 0.180 |
| Risk factor | |||
| Any risk factor | 144 (12.3) | 122 (12.1) | 0.896 |
| Indwelling urethral catheter | 6 (0.51) | 8 (0.79) | 0.435 |
| Prostate volume ≥ 75 ml | 76 (6.51) | 51 (5.06) | 0.169 |
| Dysuria (residual urinary volume ≥ 100 ml) | 69 (5.91) | 57 (5.65) | 0.854 |
| Diabetes mellitus (HbA1c ≥ 8.0%) | 12 (1.03) | 8 (0.79) | 0.656 |
| Corticosteroid use | 7 (0.60) | 12 (1.19) | 0.168 |
The incidence of ICs is summarized in Table 2 . Although the incidence of febrile UTI was lower in the CMZ+LVFX group (0.20%, 2/1,008) than in the CEZ+LVFX group (0.77%, 9/1,168), the difference was not statistically significant (95% confidence interval (95%CI): 0.03–1.24, P = 0.073). In the CEZ+LVFX group, the incidence of sepsis, bacteremia, abscess, and readmission was 0.43% (5/1,168), 0.26% (3/1,168), 0.17% (2/1,168), and 0.26% (3/1,168), respectively. However, in the CMZ+LVFX group, no ICs were observed other than febrile UTIs.
| CEZ+LVFXn = 1,168 | CMZ+LVFXn = 1,008 | P -value | |
|---|---|---|---|
| Febrile UTI | 9 (0.77) | 2 (0.20) | 0.073 |
| Sepsis | 5 (0.43) | 0 (0) | 0.066 |
| Bacteremia | 3 (0.26) | 0 (0) | 0.254 |
| Abscess | 2 (0.17) | 0 (0) | 0.502 |
| Readmission | 3 (0.26) | 0 (0) | 0.254 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree