Fig. 18.1
A 62-year-old woman who presents with a rectal adenocarcinoma involving the sphincter complex. She is not a candidate for sphincter-preserving operation. The patient underwent neoadjuvant chemoradiation therapy followed by a successful abdominoperineal resection (Courtesy of Quyen D. Chu, MD, MBA, FACS)
Evaluation
The initial evaluation of a patient who presents with the above complaints includes a complete physical examination, digital rectal examination (DRE), and proctoscopy. The patient’s nutritional status should also be recorded, although unlike patients with upper gastrointestinal cancers (i.e., esophageal cancer, pancreatic cancer, gastric cancer), poor nutrition is often not observed in patients with CRC. If present, this would suggest advanced disease.
Abdominal examination may reveal enlarged liver suggestive of liver metastasis, ascites indicative of intra-abdominal carcinomatosis, and nodules in the umbilicus or abdominal wall that may represent Sister Mary Joseph nodule. The presence of inguinal adenopathy may be suggestive of metastatic disease. On DRE, if a rectal mass is palpated, location, size, mobility, and distance of the distal edge from the anal verge or anorectal ring are assessed. The integrity and adequacy of the anal sphincter complex are evaluated. Knowing the length of one’s own examining digit is helpful in estimating the distance of the cancer from the anal verge. Information on DRE is helpful in ordering appropriate imaging studies and designing treatment plan. A small tumor within 8 cm from the anal verge may be considered for a transanal excision, while a large fixed tumor is best treated with neoadjuvant chemoradiation (neoCRT) and radical resection. In a male, a large anterior tumor that appears to involve the prostate may require a concomitant prostatectomy at the time of a definitive operation, especially if the tumor has not regressed significantly following neoadjuvant therapy. Similarly, in a female, anterior involvement of the posterior vagina might necessitate a partial vaginectomy with reconstruction. Patients with fecal incontinence and compromised anal sphincter function or integrity are not candidates for sphincter-sparing operation (Fig. 18.1). Patients presenting with obstructive symptoms may be candidates for an endoluminal stent or a diverting colostomy, the latter of which can be performed laparoscopically.
Proctoscopy is important not only to accurately visualize and localize the lesion but also to help determine its gross appearance, size, and distance from the anal verge and obtain biopsies. Morphologic features help in prognosticating patients with small rectal cancers who may be candidate for local excision. Ulcerated or flat raised tumors (nonexophytic) tend to have a worse prognosis than those that are polypoid or sessile (exophytic) lesions [4, 5]. Biopsies determine the type of tumor (adenocarcinoma, squamous cell carcinoma, small cell carcinoma, undifferentiated carcinoma, etc.) and histopathological features that are essential in the selection of small tumors for local versus local + adjuvant versus radical resection.
As part of the complete work-up, a colonoscopy is warranted to exclude synchronous lesions in the colon and determine the proximal extent of the tumor. Synchronous benign polyps occur in 13–62 % of cases, while synchronous cancers occur in 2–8 % of cases [6–8]. The rectum can be divided into thirds: upper, middle, and lower thirds. Such division can be based on the valves of Houston or by dividing the rectum into thirds using either the 12 cm or 15 cm as the cutoff point (Fig. 18.2). The National Cancer Institute Guidelines for Colon and Rectal Cancer defined the most proximal boundary of the rectum as no more than 12 cm from the anal verge [9], while the European Society for Medical Oncology (ESMO) defined rectal tumors as those with distal extension < 15 cm from the anal verge [10]. Establishing which thirds of the rectum to which the tumor belongs is essential in determining the treatment modality and type of operation the patient will ultimately require.
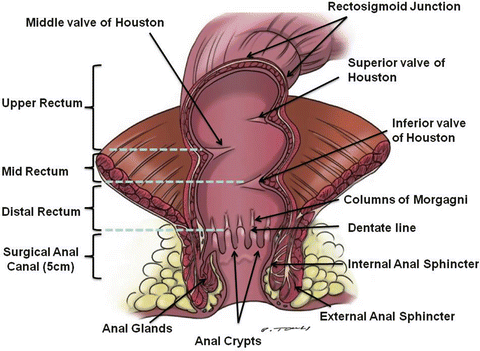

Fig. 18.2
Anatomy of the rectum: the rectum can be divided into thirds: upper, middle, and lower thirds. Such division can be based on the valves of Houston or by dividing the rectum into thirds using either the 12 cm or 15 cm as the cutoff point (Illustrated by Paul Tomljanovich, MD and modified by Karen Howard and Lory Tubbs. Courtesy of Quyen D. Chu, MD, MBA, FACS)
Unlike colon cancer which drains mainly into the portal venous system (route that leads to metastasis to the liver), rectal cancer drains into both the portal venous system and the systemic circulation, the latter via the inferior and middle rectal veins to the iliac veins and finally to the inferior vena cava. Consequently, rectal cancer can bypass the portal venous system to reside in the lungs, which explains why the incidence of isolated lung metastases is higher in rectal cancer (12 % vs. 6 %) than it is in colon cancer [11, 12]. Given rectal cancer’s affinity to metastasize to the lungs, it is reasonable to obtain CT scans of the chest as part of the work-up of patients with rectal cancer.
Imaging Modalities
Computed tomography (CT) scanning, magnetic resonance imaging (MRI), and transrectal ultrasound (TRUS) have all been extensively evaluated in the initial staging of rectal carcinoma [13–15]. Pretreatment imaging to accurately stage the patient is extremely important because it can potentially alter treatment and affect patients’ outcome. For a patient with a low-lying T1 lesion who is concerned about having a permanent colostomy and/or sexual dysfunction, a local rectal excision may be an option. However, the same patient with a T2 lesion will likely be treated with radical surgery, which may result in a permanent colostomy and/or sexual dysfunction. Thus, these decisions cannot be made without accurate presurgical staging. Although reports suggest that MRI and TRUS are better at staging rectal cancer than CT, to date, they have not been reliable enough to be used routinely as the sole imaging modality [16, 17]. Furthermore, because of the prohibitive cost associated with MRI, such modality may not be widely available in many centers.
Computed Tomography
CT was the first “staging” modality used to evaluate rectal cancer (Figs. 18.3 and 18.6a). Early enthusiasms reported its accuracy to range between 85 % and 90 % [18], and it was championed to be an excellent preoperative staging modality for characterizing both tumor and metastases. CT is still recommended in the initial evaluation of all patients scheduled for colorectal carcinoma surgery because of its ability to obtain a rapid global evaluation of the extent of disease and helpful in revealing complications (perforation, obstruction) that may not be clinically apparent. Larger, more carefully controlled studies, however, have shown that the overall accuracy of CT is in the 50–70 % range, varying directly with the stage of the lesion (i.e., T4 lesions are more accurately assessed than T2 or T3 lesions) [13, 15, 19]. Overstaging is a far more common problem as it is difficult to accurately determine T-stage (depth of bowel wall penetration) on CT [20]. Another complicating factor, particularly in rectal cancer, is that perirectal spiculation can be confused with desmoplastic peritumoral inflammation, which can lead to overstaging.
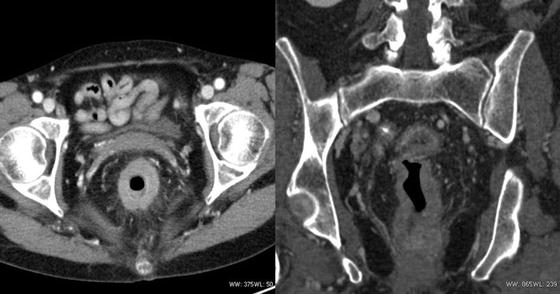

Fig. 18.3
A 64-year-old woman with an advanced rectal cancer. She underwent neoadjuvant chemoradiation therapy and a laparoscopic abdominoperineal resection (Courtesy of Quyen D. Chu, MD, MBA, FACS)
There is little agreement on the critical cutoff diameter to determine if lymph nodes are involved. One study suggests that the cutoff value should be 4.5 mm; however, nodal size does not correlate with nodal status [21, 22]. The specificity for detecting lymph nodes involved with tumor is only 45 % [23].
Liver metastases are detected by CT with an 85 % accuracy rate and a 97 % specificity rate [15]. Detection of liver metastases by CT improves with increasing stage of disease. Among a group of 100 patients who underwent CT, CT arterioportography (CTAP), and MRI, the sensitivity and specificity for liver metastases were 73 % and 96.5 % for CT, 87.1 % and 89.3 % for CTAP, and 81.9 % and 93.2 % for MRI [19]. In addition, abdominal/pelvic CT has a high negative predictive value of 90 % [21].
As mentioned previously, rectal cancer can spread to the lungs via the systemic portal circulation route. Among patients with potentially resectable liver metastases and a negative initial chest radiograph, additional imaging with a chest CT revealed pulmonary metastases in only 5 % of patients [24]. However, one study showed that rectal cancer is more likely than colon cancer to present with lung metastases without liver metastases and that this risk increases with advancing T-stage. Although this study advised CT imaging of the chest in all rectal cancer patients, it was limited by the lack of pathologic correlation [25].
Magnetic Resonance Imaging
The Radiology Diagnostic Oncology Group (RDOG) study showed that MRI had an accuracy rate of 58 % for detecting local staging of rectal cancer [15] (Fig. 18.4). Accuracy in identifying lymph node metastases was similar to CT with a sensitivity of 85 %, but MRI was slightly superior for detecting liver metastases. Endorectal MR coils and 3.0 T magnets [27] have shown impressive results in depicting the layers of the rectal wall with resultant improvement in the accuracy of assessing the depth of bowel wall penetration [17]. There is no consensus in the literature as to whether endorectal coils should be used routinely in practice. Some studies contend that endorectal coils provide improved diagnostic accuracy as compared to phased-array coils alone for T-stage, with sensitivity reaching 100 % and specificity of 86 %. Endorectal coils have limitations in assessing upper rectal tumors and lateral pelvic and inferior mesenteric lymph nodes. Although phased-array coils are far superior in detecting lymph node metastases, they are limited in the imaging of obese patients and in the evaluation of lower rectal tumors [28, 29]. With the advent of 3.0 T imaging, most imaging can be performed with a pelvic phased-array coil only.
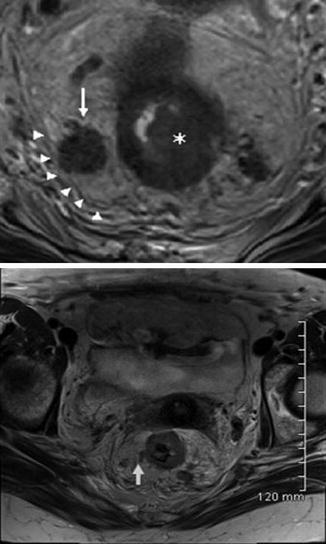

Fig. 18.4
MRI of the rectum, high‐resolution magnetic resonance imaging of rectal cancer used for staging. (Top) An axial, T2‐weighted, nonfat‐saturated image demonstrating a rectal tumor identified invading the muscularis propria. There is an adjacent enlarged heterogenous lymph node (arrow) in contact with the mesorectal fascia (arrow heads). Incidental note is made of another heterogenous lymph node to the left of the rectum. (Bottom) Vascular invasion in the linear area of an abnormal T2 signal extending from the tumor margin (arrow) (Reprinted from Ref. [26]. With permission from John Wiley & Sons, Inc.)
MRI is accurate at identifying high-risk features such as extramural venous invasion, extramural spread beyond 5 mm, and potentially positive circumferential resection margin (CRM). CRM, which is the distance of the leading edge of the tumor to the mesorectal fascia, is an important prognostic factor for determining risk of local recurrence. The accuracy, sensitivity, and specificity of MRI to predict CRM involvement are 86 %, 94–100 %, and 85–88 %, respectively [30]. From a surgical perspective, assessment of the mesorectal fascia involvement and tumor-free CRM is crucial for surgical planning [31].
Diffusion-weighted imaging (DWI) has shown to be more sensitive and specific than standard contrast-enhanced MRI with gadolinium-enhanced MRI, with values of 82 % and 94 %, respectively [32]. It is believed to be superior for tumor detection and characterization and for monitoring tumor response. Adding DWI to conventional MRI yields better diagnostic accuracy than conventional MRI alone [32]. DWI does not use contrast and is more sensitive than contrast-enhanced CT in detecting metastases [33]. It also has the potential to be clinically effective for the evaluation of preoperative TNM staging and the postoperative follow-up of colorectal cancer.
Transrectal Ultrasound
TRUS has become the standard imaging procedure for staging rectal carcinoma [34]. Because TRUS enables one to distinguish the layers within the rectal wall, it is an accurate method for detecting depth of tumor penetration and perirectal spread. Reported sensitivities range between 83 % and 97 %. The T-stage accuracy for TRUS (84.6 %) is far superior to that of CT (70.5 %) [22]. TRUS is of value in assessing apparently superficial rectal carcinomas that are potentially suitable for treatment by transanal or local excision or endocavitary radiation [23]. Detection of lymph node involvement with TRUS is difficult; the sensitivity rate is 50–57 % [29] and the overall accuracy is between 62 % and 83 % [35]. Although TRUS is frequently used to assess regional lymph nodes, it is not very reliable in predicting actual nodal involvement. Many lymph nodes measuring <5 mm in diameter have associated micrometastases, while some early stage T1 and T2 tumors that have lymph node micrometastases were missed by TRUS. Such limitations partly explain the high pelvic recurrence rate observed within this patient population who underwent local rectal excision.
Nuclear Medicine
Positron emission tomography (PET) and PET/CT have been shown to alter therapy in almost a third of patients with advanced primary rectal cancer. In a study comparing PET/CT with TRUS, MRI, and helical CT in imaging patients with low rectal carcinoma, PET/CT identified discordant findings and was far superior in 38 % of patients. The result was an upstaging in half the patients while downstaging in almost a quarter of patients (21 %) [36]. A relatively new concept of using PET/CT has been reported to be significantly more accurate in defining TNM stage than CT alone [36]. However, it is not used routinely in most centers. The accuracy of PET/CT is similar to that of CT in terms of T-stage but is far superior in detecting hepatic and peritoneal metastases (sensitivity, 89 %, and specificity, 64 %) [37]. The sensitivity of detecting nodal metastases is only 43 % with a specificity of 80 %, and again, size is not a helpful characteristic. There is also a potential role for PET in restaging colorectal cancer after chemoradiotherapy by measuring the pretreatment and posttreatment standard uptake volume (SUV) and assessing response by the amount of decreasing SUV [38]. Limitations of PET include decreased sensitivity in detecting small colonic lesions (5–10 mm in diameter) and decreased 18F-fluorodeoxyglucose (FDG) uptake by mucinous tumors.
Staging
Clinical stage of rectal cancer is determined by preoperative DRE and imaging studies (CT scan, ERUS, endorectal coil MRI, and/or PET scan). Pathologic staging is determined after excision of the cancer. Rectal cancer is staged using the seventh edition of the American Joint Committee on Cancer (AJCC)/TNM staging system (Table 18.1) [42]. By definition, stage II has uninvolved nodes, whereas stage III has involved lymph nodes. There are several important changes made to the sixth edition. T4 lesions are subdivided into T4a (tumor penetrates to the surface of the visceral peritoneum) and T4b (tumor directly invades or is adherent to other organs or structures). The number of involved lymph nodes also has an impact on outcomes; thus, N1 is subdivided into N1a (metastasis in 1 regional lymph node), N1b (metastasis in 2–3 lymph nodes), and N1c (no nodal disease, but tumor deposits in the subserosa, mesentery, or non-personalized pericolic or perirectal tissues); N2 is subdivided into N2a (metastasis in 4–6 nodes) and N2b (metastasis in ≥ 7 nodes) [40].
Table 18.1
American Joint Committee on Cancer (AJCC) TNM staging for colorectal carcinoma (7th edition)
Primary tumor (T)* | |
|---|---|
TX | Primary tumor cannot be assessed |
T0 | No evidence of primary tumor |
Tis | Carcinoma in situ: intraepithelial or invasion of lamina propria* |
T1 | Tumor invades submucosa |
T2 | Tumor invades muscularis propria |
T3 | Tumor invades through muscularis propria into pericolorectal tissues |
T4a | Tumor penetrates to the surface of the visceral peritoneum** |
T4b | Tumor directly invades or is adherent to other organs or structures**, *** |
*Note: Tis includes cancer cells confined within the glandular basement membrane (intraepithelial) or mucosal lamina propria (intramucosal) with no extension through the muscularis mucosae into the submucosa | |
**Note: Direct invasion in T4 includes invasion of other organs or other segments of the colorectum as a result of direct extension through the serosa, as confirmed on microscopic examination (e.g., invasion of the sigmoid colon by a carcinoma of the cecum) or, for cancers in a retroperitoneal or subperitoneal location, direct invasion of other organs or structures by virtue of extension beyond the muscularis propria (i.e., respectively, a tumor on the posterior wall of the descending colon invading the left kidney or lateral abdominal wall or a mid or distal rectal cancer with invasion of prostate, seminal vesicles, cervix, or vagina) | |
***Note: Tumor that is adherent to other organs or structures, grossly, is classified cT4b. However, if no tumor is present in the adhesion, microscopically, the classification should be pT1–4a depending on the anatomical depth of wall invasion. The V and L classifications should be used to identify the presence or absence of vascular or lymphatic invasion, whereas the PN site-specific factor should be used for perineural invasion | |
Regional lymph nodes (N) | |
|---|---|
NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph node metastasis |
N1 | Metastasis in 1–3 regional lymph nodes |
N1a | Metastasis in 1 regional lymph node |
N1b | Metastasis in 2–3 regional lymph nodes |
N1c | Tumor deposit(s) in the subserosa, mesentery, or nonperitonealized pericolic or perirectal tissues without regional nodal metastasis |
N2 | Metastasis in ≥4 regional lymph nodes |
N2a | Metastasis in 4–6 regional lymph nodes |
N2b | Metastasis in ≥7 regional lymph nodes |
Note: A satellite peritumoral nodule in the pericolorectal adipose tissue of a primary carcinoma without histologic evidence of residual lymph node in the nodule may represent discontinuous spread, venous invasion with extravascular spread (V 1/2), or a totally replaced lymph node (N1/2). Replaced nodes should be counted separately as positive nodes in the N category, whereas discontinuous spread or venous invasion should be classified and counted in the site-specific factor category tumor deposits (TD) | |
Distant metastasis (M) | |
|---|---|
M0 | No distant metastasis |
M1 | Distant metastasis |
M1a | Metastasis confined to one organ or site (e.g., the liver, lung, ovary, nonregional node) |
M1b | Metastases > one organ/site or the peritoneum |
Anatomical stage/prognostic groups | |||||
|---|---|---|---|---|---|
Stage | T | N | M | Dukes* | MAC* |
0 | Tis | N0 | M0 | – | – |
I | T1 | N0 | M0 | A | A |
T2 | N0 | M0 | A | B1 | |
IIA | T3 | N0 | M0 | B | B2 |
IIB | T4a | N0 | M0 | B | B2 |
IIC | T4b | N0 | M0 | B | B3 |
IIIA | T1–2 | N1/N1c | M0 | C | C1 |
T1 | N2a | M0 | C | C1 | |
IIIB | T3–T4a | N1/N1c | M0 | C | C2 |
T2–T3 | N2a | M0 | C | C1/C2 | |
T1–T2 | N2b | M0 | C | C1 | |
IIIC | T4a | N2a | M0 | C | C2 |
T3–T4a | N2b | M0 | C | C2 | |
T4b | N1–N2 | M0 | C | C3 | |
IVA | Any T | Any N | M1a | – | – |
IVB | Any T | Any N | M1b | – | – |
Note: cTNM is the clinical classification; pTNM is the pathologic classification. The y prefix is used for those cancers that are classified after neoadjuvant pretreatment (e.g., ypTNM). Patients who have a complete pathologic response are ypT0N0cM0 that may be similar to stage group 0 or I. The r prefix is to be used for those cancers that have recurred after a disease-free interval (rTNM) | |||||
*Dukes B is a composite of better (T3N0M0) and worse (T4N0M0) prognostic groups, as is Dukes C (any TN1M0 and any TN2M0). MAC is the modified Astler-Coller classification | |||||
In the seventh edition, stage group II was subdivided into three subdivisions instead of two: stage IIA (T3N0), IIB (T4aN0), and IIC (T4bN0). Additionally, several stage group III were reclassified (i.e., T4bN1 is reclassified as IIIC instead of IIIB, T1N2a is IIIA instead of IIIC, T1N2b, T2N2a–b, and T3N2a are IIIB instead of IIIC). Finally, M1 is subdivided into M1a (single metastatic site) and M1b (multiple metastatic sites).
Treatment
Management of rectal cancer requires a multidisciplinary approach. However, surgery remains the mainstay treatment modality of rectal cancer. The primary goals are to cure the patient, maintain function, reduce local recurrence, maximize disease-free survival (DFS), and optimize quality of life. Locoregional recurrence after surgery is related in part to surgical technique. Treatment of rectal cancer depends on location of the tumor and staging information. Selected cases of rectal cancer can be treated with local excision (discussed in Chap. 17 , Local Excision of Early-Stage Rectal Cancer); however, the majority of rectal cancers are treated with radical resection with or without adjuvant therapy.
Upper Rectal Cancer
The rectosigmoid junction is identified at the level of the promontory of the sacrum and where the teniae coli coalesce to form the continuous longitudinal muscle layer around the rectum. The anterior and lateral wall of the upper rectum is covered with peritoneum, and unlike the middle and distal rectum, it is not confined within the bony pelvis. The concern of sphincter preservation is therefore not relevant.
While it is widely accepted that combination therapy comprising of chemoradiation therapy (CRT) and surgery is the treatment of choice for patients with stage II and III rectal cancer in the mid and distal rectum, the benefit of CRT does not extend to patients with upper rectal or rectosigmoid cancers. Lopez-Kostner et al found that local recurrence and survival for upper rectal cancer were similar to those of sigmoid cancer and because of this, the upper rectum is treated more like colon cancer than rectal cancer [43]. In other words, chemoradiation and possibly total mesorectal excision (TME) may be spared in patients with upper rectal/rectosigmoid cancer, especially those that are T3N0 (Stage II). Both the Swedish Rectal Trial and Dutch Rectal Trial found that, compared to surgery alone, preoperative radiation followed by surgery had no significant impact on local recurrence rate for upper rectal tumors [44].
For upper rectal cancer, anterior resection with tumor specific rather than TME is required. The mesorectum is divided 3–5 cm below the lesion [45]. Alternatively, anterior resection without TME for upper rectosigmoid cancer is also an acceptable option.
Mid and Distal Rectal Cancer
Unlike the colon, the mid and distal rectum are confined in the pelvis, constrained by the bony pelvis, surrounded by major neurovascular structures, and in close proximity to urogenital organs which makes it difficult to achieve a wide resection around the tumor. Consequently, the risk of local recurrence is higher than with colon cancer. Also, unlike colon cancer, adjuvant radiation is required with stage II rectal cancer.
For the majority of rectal cancers, radical or major surgical resection is optimal, sphincter-sparing operation such as a low or ultralow anterior resection (LAR or ULAR) with or without reconstruction for mid to low rectal cancer, and an abdominoperineal resection (APR) for some mid rectal cancers and all low rectal cancer (Fig. 18.5). Better stapling devices have increased the success of low anastomoses, thus allowing more liberal use of sphincter-sparing surgeries. The decision to perform an LAR versus an APR depends mainly on whether an adequate distal margin can be achieved and whether the sphincter complex is compromised. With LAR, the colorectal anastomosis is performed hand sewn or stapled. To perform ULAR without reconstruction, a straight low rectal or coloanal anastomosis is performed either with hand sewn at the dentate line after excision of the columnar to the level of the anorectal ring or stapled at or just above the level of the anorectal ring. For ULAR with construction, the neorectum is fashioned (transverse coloplasty or colonic J-pouch) and anastomosed to the low rectum or just above the anorectal ring. With reconstruction, the functional outcome is superior to straight coloanal or low colorectal anastomosis [47, 48]. Other procedures that involve posterior or intersphincteric resection are not widely used and will not be further discussed. Kraske transsacral and York-Mason transphincteric resections are of historic interest. Transabdominal coloanal anastomosis after removal part of or the entire internal sphincter (intersphincteric resection) is associated with high positive circumferential margin.
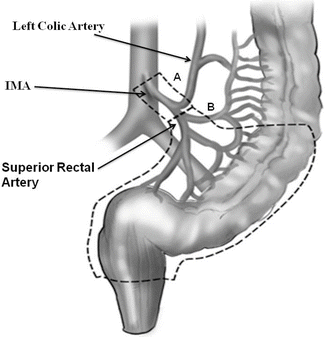

Fig. 18.5
Surgical approaches to rectal cancer. In addition to resecting sufficient segment of bowel proximal and distal to the tumor and mesorectal excision, the major feeding vessel accompanied by lymphatics and LN are ligated at the level of their origin from the aorta. The inferior mesenteric artery (IMA) close to the aorta is taken (high ligation, Fig. 18.5A), but ligation above the bifurcation of the aorta distal to the left colic artery is sufficient (low ligation at the level of the superior rectal artery, Fig. 18.5B) (Reprinted from Ref. [46]. Available from http://www.intechopen.com/books/cancer-treatment-conventional-and-innovative-approaches/current-strategies-in-the-management-of-adenocarcinoma-of-the-rectum with permission from InTech)
Mortality of rectal cancer is related to metastatic disease present prior to surgery and recurrence after surgery. Local recurrence is related to technique and adequacy of resection. Pelvic recurrence poses a management dilemma because the morbidity is quite substantial and the majority of patients who recurred are not amenable to a reoperation. The high LR rate observed in the past is likely due to suboptimal surgical techniques as well as the medical community’s lack of knowledge about the biology of the disease.
The goal of radical or major resection is to (1) obtain adequate clearance around the tumor and tumor-free resection margins (proximal, distal, and circumferential), (2) remove LN-bearing mesorectum with an intact envelope, (3) ligate the IMA at its origin, (4) harvest at least ≥12 regional LN, (5) minimize the risk of tumor perforation or rupture, and (6) en bloc resection of any adherent structure.
Margins
Surgery requires resection of a sufficient segment of bowel proximal and distal to the tumor. Generally, proximal margin ≥ 5 cm is sufficient and 2 cm for distal margins is ideal. However, for distal rectal cancers a 1–2 cm is acceptable. Appreciable distal intramural spread of rectal adenocarcinoma is noted in only 25 % of cases and is almost always within 1.5 cm of the primary tumor. Tumor spread > 1.5 cm is found in poorly differentiated or widely metastatic cancer. In the era of neoCRT, the required 2 cm distal margin can be reduced to 1 cm [49]. To reiterate, patients whose tumors that cannot be safely resected with a clear distal margin or have evidence of sphincter involvement or dysfunction should undergo an APR. Inadequate number of lymph nodes retrieved is associated with increased mortality in both node-negative and node-positive rectal cancer [50, 51].
Circumferential margin is the nonperitonealized surface of the rectal specimen created by mesorectal dissection at surgery (Fig. 18.6c). Circumferential margin is considered positive if the distance between the deepest extent of the tumor and closed surgical clearance around the tumor, i.e., circumferential resection margin (CRM), is 0 to 1 mm. In radical resection, a > 1 mm circumferential resection margin (CRM) is optimal. CRM has been found to be an independent predictor of outcome in patients with rectal cancer. An involved CRM is defined as tumor within or equal to 1 mm or less from the CRM [52]. When CRM is < 1 mm, local recurrence rate was 22 %, but when it was more than 1 mm, this rate drops to 5 % [53]. Furthermore, CRM < 1 mm was predictive of an increased risk of distant metastases (37 % vs. 15 % for those with CRM > 1 mm) and shorter survival (70 % vs. 90 % at 2 years for those with CRM > 1 mm) [54]. However, other investigators have considered 2 mm as the cutoff point [55]. Nagtegaal reported that the local recurrence was 16 % for CRM <2 mm versus 6 % for patients with radial margins > 2 mm [55]. Although the ideal CRM has not been universally accepted, the general principle is that the operating surgeon should strive for as wide of a CRM margin as possible.
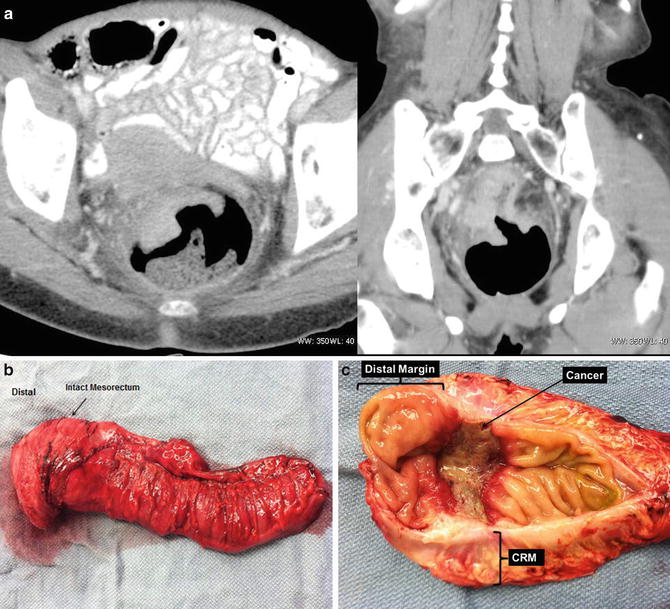

Fig. 18.6
A 67-year-old woman with a rectal cancer located approximately 5 cm from the anal verge. CT demonstrated an advanced rectal cancer (a). She underwent preoperative chemoradiotherapy, followed by a low anterior resection, total mesorectal excision, and a diverting loop ileostomy (b). The final pathology demonstrated a T3N0M0 disease. None of the 16 lymph nodes were involved; the distal margin was 3 cm from the tumor and the circumferential margin was 8 mm (c) (Courtesy of Quyen D. Chu, MD, MBA, FACS)
Mesorectal Excision
Radical surgery also requires resection of the mesorectum that contains fat, LN (regional LN), and the lymphatics. Heald et al described total mesorectal excision (TME) that involves complete removal of the LN-bearing mesorectum along with its intact enveloping fascia [56]. TME requires sharp dissection, instead of the conventional blunt dissection, in the extrafascial plane between the presacral fascia and the fascia propria of the rectum. TME requires the complete excision of the visceral mesorectal tissue not only the proximal mesorectum but also the distal mesorectum down to the level of the levators [57, 58] (Figs. 18.6b, c and 18.7a–c). TME does require intense training, but when done properly, it can result in substantial locoregional control in long-term outcomes for patients with rectal cancer. Proper identification and preserving branches of the hypogastric nerves innervating the pelvic organ can spare the patient the sequelae of urinary retention and sexual dysfunction (retrograde ejaculation). TME results in a higher number of lymph nodes retrieved because the lymph node bearing the mesorectum is resected. Finally, TME accomplishes adequate circumferential radial margin (CRM) (Fig. 18.6b, c.)
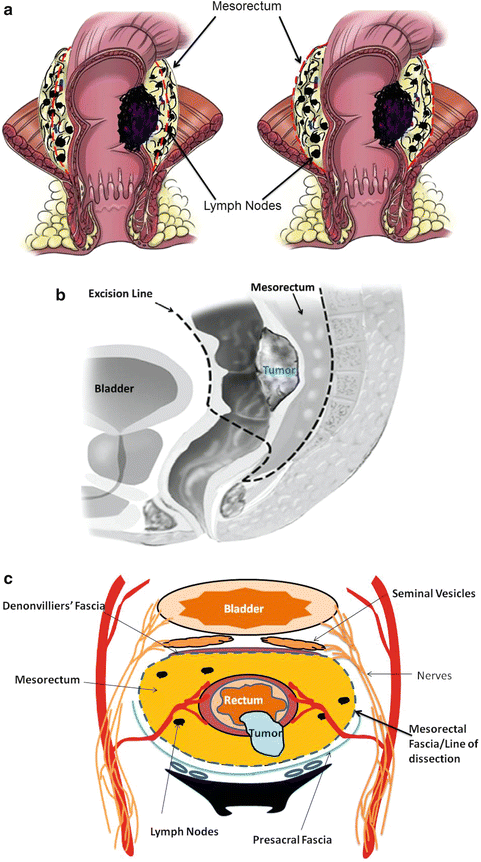

Fig. 18.7
(a) Total mesorectal excision (TME): TME requires resection of the mesorectum that contains fat, LN (regional LN), and the lymphatics, along with its intact enveloping fascia. Panel A of Fig. 18.7a demonstrates inadequate TME when the line of resection (red dotted lines) does not incorporate the mesorectum. Panel B of Fig. 18.7a depicts an adequate TME that incorporates the lymph node-bearing mesorectum. (b) Sagittal view of a male pelvis: the mesorectum is resected at least 5 cm below the tumor. Dotted lines depict extent or resection to achieve sufficient TME. (c) Cross section showing TME and the relationship with other pelvic structures: care should be taken to avoid injury to the hypogastric nerve. (a: Illustrator-Paul Tomljanovich, MD, modified by Karen Howard and Quyen D. Chu, MD; Courtesy of Quyen D. Chu, MD, MBA, FACS) (b: Reprinted from Ref. [46]. Available from http://www.intechopen.com/books/cancer-treatment-conventional-and-innovative-approaches/current-strategies-in-the-management-of-adenocarcinoma-of-the-rectum with permission from InTech.) (c: Courtesy of Quyen D. Chu, MD, MBA, FACS)
Vascular Pedicle
In addition to resecting sufficient segment of bowel proximal and distal to the tumor and mesorectal excision, the major feeding vessel accompanied by the lymphatics and LN are ligated at the level of their origin from the aorta. The inferior mesenteric artery (IMA) close to the aorta is taken, but ligation above the bifurcation of the aorta distal to the left colic artery is sufficient (Figs. 18.5 and 18.8). Along with mobilization of the splenic flexure, the inferior mesenteric vein (IMV) may be ligated at the lower edge of the pancreas to allow the colon to reach the pelvis so as to construct a tension-free anastomosis. The IMV can be identified after taking down the ligament of Treitz; it is located just to the left of the ligament.
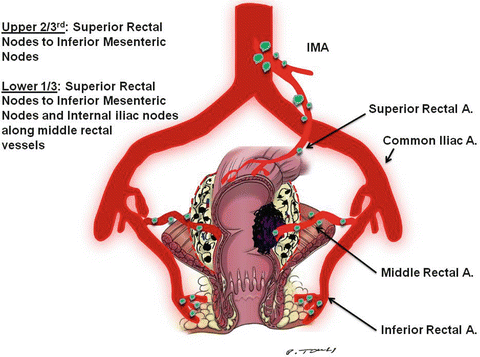

Fig. 18.8
Lymph nodes of the rectum. Note that the upper 2/3 of the rectum is drained by lymph nodes along the superior rectal artery to the lymph nodes along the inferior mesenteric artery (IMA). The lower 1/3 of the rectum is drained by lymph nodes along the superior rectal artery to the lymph nodes along the IMA. It also drains into the lymph nodes along the middle rectal artery, which drains into the nodes along the internal iliac artery and then to the nodes along the para-aortic LN. (Illustrator-Paul Tomljanovich, MD, modified by Karen Howard and Quyen D. Chu, MD; Courtesy of Quyen D. Chu, MD, MBA, FACS)
Lymph Node Dissection
For adequate staging of rectal cancer, at least ≥12 regional lymph nodes, as recommended by the American College of Surgeons, the American College of Pathology, the National Comprehensive Cancer Network (NCCN), and the American Association of Clinical Oncology (ASCO) for colorectal cancers [40, 59–61], must be harvested.
The lymphatics from the rectum drain to the mesorectal LN then upward along the superior rectal artery (or superior hemorrhoidal artery, SHA) toward the mesenteric LN along the IMA to the lateral aortic and para-aortocaval LN (Figs. 18.5 and 18.8). Drainage into paracolic LN is unusual. From the middle and lower rectum, there are two pathways for lymphatic drainage: upward along SHA and laterally to lateral pelvic LN. Downward spread is uncommon. Lateral drainage occurs to intermediate lateral LN (LN along the middle hemorrhoidal artery outside fascia propria) and lateral main LN (along internal iliac artery and obturator artery) to para-aortic LN. The lower rectum however has a cloacal origin and its lymphatic channels are part of the pedicles draining to lateral LN. The number of LN found in the mesorectum ranges from 14 to 28 depending on the method of preparation of specimens. The majority of the mesorectal LNs are located posteriorly with few on each side. There are relatively few LNs in the mesorectum of the lower rectum. Most of the LNs (70 %) are found around the branches of the SHA proximal to the peritoneal reflection, and 30 % are found distal to the peritoneal reflection. In surgical terms, the lymphatic spread of cancer occurs to perirectal (mesorectal LN) and upward along the IMA. With mesorectal excision and ligation of the IMA close to its origin, enough LN will be harvested. Lateral spread to lateral LN is more clinically important in tumors with lower margin below 5 cm from the dentate line, and the incidence becomes significantly higher with lower margin below 3 cm above the dentate line. Spread from the lower rectum laterally to the iliac LN occurs in about 15 % of cases. Lateral spread occurs to LN along the middle rectal artery that lies outside the fascia propria. More extensive nodal dissection such as lateral LN dissection that removes nodal tissue along the common and internal iliac artery demonstrated no survival benefits but increased morbidity (18 % urinary dysfunction, 50 % sexual dysfunction) and therefore should not be performed [62–64]. To reiterate, a TME should be a part of the surgical operation, especially for tumors in the middle and lower rectum.
Tumor Rupture and En Bloc Resection
Inadvertent rupture of the tumor during dissection is associated with increase in LR and decrease in 5-year survival. Separation of structure adherent to the tumor is considered incomplete resection and associated with adverse outcome [9].
Adjuvant and Neoadjuvant Therapy
Patients with early stage rectal cancer (T1-2, N0, M0, or stage I disease) enjoy a 5-year survival rates greater than 90 % after radical surgery alone, and therefore, adjuvant or neoadjuvant therapy is not necessary. For patients with clinical stage II (cT3–4, N0, M0) and III (any T, N+, M0) diseases according to the AJCC/TNM and International Union Against Cancer (IUCC), multimodality therapy (chemotherapy, radiotherapy, surgery) is the treatment of choice. However, this has not always been the case. Historically, surgery alone in this high-risk group resulted in an unacceptably high rate of local recurrence (LR); pelvic recurrence occurs in approximately 30–65 % when surgery alone was performed [65–69].
Pelvic recurrence poses a management dilemma because the morbidity is quite substantial and the majority of patients who recurred are not amenable to a reoperation. The high LR rate observed in the past was likely due to suboptimal surgical techniques (the use of blunt dissection rather than sharp dissection) as well as the paucity of knowledge about the natural history of the disease.
In 1985, the Gastrointestinal Tumor Study Group (GITSG), spearheaded by members of Roswell Park Cancer Institute, Buffalo, New York, conducted a randomized trial to assess the efficacy of postoperative chemoradiation (postCRT) in a group of high-risk patients who underwent “curative” rectal resection [70, 71] (Table 18.2). This landmark study altered the landscape of the management of rectal cancer because it established that adjuvant therapy was better than surgery alone; combination of postoperative chemotherapy (5-fluorouracil or 5-FU based) and radiation therapy significantly reduced recurrence rate from 55 % in the surgery alone group to 33 % in the postCRT group (P < 0.04). A subsequent study comparing postoperative radiation alone with postCRT confirmed the superiority of postCRT [73]. In this study, postCRT not only significantly reduced rectal recurrence by 34 % (P = 0.0016) but also reduced cancer-related deaths by 36 % (P = 0.0071) and overall deaths by 29 % (P = 0.025).
Table 18.2
Summary of selected trials on adjuvant and neoadjuvant treatment of rectal adenocarcinoma
Authors, year | N | Median follow-up (mos) | Treatment groups | TME performed? | Outcome |
|---|---|---|---|---|---|
202 | 80 | 1. Adjuvant chemoXRT | No | Adjuvant chemoXRT significantly reduces LR rate from 55 % to 33 % (P < 0.04) | |
2. Observation | |||||
NSABP R-01, 1988 [72] (Fisher) | 555 | 64 | 1. Adjuvant chemo | No | Adjuvant chemo significantly reduced DFS and OS compared to observation |
2. Adjuvant XRT | |||||
3. Observation | |||||
Krook 1991 [73] | 204 | 84 | 1. Adjuvant chemoXRT | No | Adjuvant chemoXRT reduces |
2. Adjuvant XRT | Recurrence by 34 % (P = 0.0016) | ||||
Cancer-related deaths by 36 % (P = 0.0071) | |||||
Overall deaths by 29 % (P = 0.025) | |||||
Frykholm 1993 [74] | 471 | 60 | 1. Preoperative SC XRT | No | LR lower in preoperative group (13 % vs. 22 %; P = 0.02) |
2. Postoperative LC XRT | |||||
Overall survival: no difference | |||||
Morbidity: no difference | |||||
1168 | 156 | 1. Preoperative SC XRT | No | Only trial that demonstrated survival benefit with preoperative XRT | |
2. Observation | |||||
Colorectal Cancer Collaborative Group 2001 [77]* | 8507 | NA | 1. Preoperative XRT | NA | Overall survival: no differences |
2. Postoperative XRT | 5-year LR rates | ||||
3. Observation | Preop XRT, 12.5 % | ||||
Observation, 22.2 % (P < 0.00001) | |||||
1861 | 144 | 1. Preoperative SC XRT + TME | Yes | Preop XRT significantly reduces LR rates by > 50 % relative to surgery alone | |
2. TME | Overall survival: no differences | ||||
10-year LR rates | |||||
Preop XRT/TME, 5 % | |||||
TME, 11 % (P < 0.0001) |
The National Surgical Adjuvant Breast and Bowel Project (NSABP) R01 trial reported that adjuvant 5-FU after rectal surgery was associated with improved survival compared with either surgery alone or surgery with adjuvant radiation [72]. Based on these studies, the National Institute of Health (NIH) consensus conference in circa 1990 recommended postCRT as standard treatment for patients with stage II and III rectal cancer [79]. These recommendations, however, have changed with the advent of subsequent clinical trials.
Role of Preoperative Radiation Therapy
In 1997, the Swedish Rectal Cancer Trial group reported a large phase III trial of over 1100 patients comparing a short course of preoperative radiation therapy followed by surgery (please see section on short-course versus long-course radiation therapy below) versus surgery alone and found that the preoperative radiation group not only had a significantly lower local recurrence rate (11 % vs. 27 %; P < 0.001) but also an improved 5-year overall survival (OS) rate (58 % vs. 48 %; P = 0.004) [75]. These results were demonstrated to be durable in their follow-up report (median follow-up time = 13 years [76]). In 2001, the Colorectal Cancer Collaborative Group performed a systematic review of over 8,500 patients from 22 randomized trials and found that both preoperative XRT (neoRT) and postoperative XRT (postRT) were effective at decreasing local recurrence rate when compared to surgery alone [77]. Of note, while multiple subsequent rectal cancer trials by other investigators demonstrated an improved local control rate, the Swedish trial is the only one thus far that had demonstrated an additional OS benefit with combination therapy (Table 18.2).
Total Mesorectal Excision (TME) Era
Almost all of the above earlier studies did not emphasize the importance of surgical techniques in reducing locoregional recurrences. The Dutch Colorectal Cancer Group was the first to assess the role of TME in controlling LR [44]. Over 1800 patients with resectable rectal cancer were randomized to either a short course of neoRT followed by TME or TME alone. Although 2-year OS was not significantly different between the groups (82 % vs. 81.8 %; P = 0.84), the rate of local recurrence at 2 years was significantly reduced in the neoRT/TME group (2.4 % vs. 8.2 %; P < 0.001) [44]. The 12-year follow-up results confirmed a 10-year cumulative incidence of local recurrence of 5 % in the neoRT/TME group versus 11 % in the TME alone group (P < 0.0001) [78] (Table 18.2). The Dutch study galvanized the importance of TME in the management of patients with rectal cancer, which became standard procedure in subsequent clinical trials.
Preoperative Chemoradiotherapy Versus Preoperative Radiotherapy Alone
Although neoRT was effective at controlling local disease, significant tumor downsizing rarely occurred, especially when short-course radiation regimen is used. To improve tumor response, several investigators evaluated the efficacy of adding chemotherapy to neoadjuvant radiation (neoCRT) [80–85] (Table 18.3).
Table 18.3
Selected randomized trials comparing preoperative chemoradiotherapy with preoperative radiotherapy alone in resectable rectal cancer
Authors/year | N | Median follow-up (mos) | Outcome | Summary |
|---|---|---|---|---|
Boulis-Wassif 1984 [80] | 247 | 84 | 5-year OS | NeoCRT had borderline significance in OS |
NeoCRT, 59 % | ||||
PreRT, 46 % (P = 0.06) | ||||
5-year LR rates | ||||
NeoCRT, 85 % | ||||
PreRT, 85 % | ||||
Bosset 2014 [86] (EORTC 22921) | 1011 | 125 | 10-year OS | LR significantly higher in preRT |
NeoCRT, 50.7 % | No differences in | |||
PreRT, 49.9 % (P = 0.91) | OS, DFS, distant metastases rate | |||
10-year DFS | Long-term side effects | |||
NeoCRT, 46.4 % | ||||
PreRT, 44.2 % (P = 0.38) | ||||
10-year LR rate | ||||
NeoCRT, 11.8 % | ||||
PreRT, 22.4 % (P = 0.0017) | ||||
316 | 48 | 4-year OS | No differences in | |
NeoCRT, 66.2 % | OS, DFS, LR rate | |||
PreRT, 67.2 % (P = 0.96) | Incidence of distant metastases | |||
4-year DFS | Late toxicity | |||
NeoCRT, 55.6 % | ||||
PreRT, 58.4 % (P = 0.82) | ||||
LR rate | ||||
NeoCRT, 14.2 % | ||||
PreRT, 9 % (P = 0.17) | ||||
Gerard 2006 [83] (FFCD 9203) | 742 | 81 | 5-year OS | Grade 3 and 4 toxicity higher in neoCRT |
NeoCRT, 67.4 % | No differences in | |||
PreRT, 67.9 % (P = 0.684) | ||||
Sphincter preservation rate | ||||
OS | ||||
LR was lower in neoCRT | ||||
5-year PFS | ||||
NeoCRT, 59.4 % | ||||
PreRT, 55.5 % (P = 0.82) | ||||
5-year LR rate | ||||
NeoCRT, 8.1 % | ||||
PreRT, 16.5 % (P = 0.004) | ||||
Latkauskas 2012 [85] | 83 | N/A | N/A | NeoCRT significantly downsizes and downstages tumor |
No difference in | ||||
R0 resection rate | ||||
Sphincter preservation rate | ||||
Postoperative morbidity rate | ||||
De Caluwé, 2013 [84] (Cochrane meta-analysis) | 2399 | N/A | 5-year OS | NeoCRT |
NeoCRT, 63.9 % | Higher toxicity with neoCRT | |||
PreRT, 65.2 % (P = 0.58) | Lower LR rate | |||
5-year DFS | No differences in | |||
NeoCRT, 57.5 % | OS, DFS | |||
PreRT, 54.9 % (P = 0.27) | Sphincter preservation rate | |||
5-year LR rate, | Postoperative mortality | |||
NeoCRT, 9.4 % | Anastomotic leak rate | |||
PreRT, 16.5 % (P < 0.001) |
A large phase III trial conducted by the European Organisation for Research and Treatment of Cancer (EORTC) found that neoCRT was better at controlling local disease than neoRT [86, 88]. EORTC 22921 randomized over 1000 patients into four groups, neoRT alone, neoCRT alone, neoRT + adjuvant chemotherapy, and neoCRT + adjuvant chemotherapy. The initial results were reported in 2006 [88] and the latest long-term results were reported in 2014 [86]. With a median follow-up of 10.4 years, the 10-year cumulative risk of local relapse was significantly higher in the preRT alone arm than in any of the three chemotherapy groups (P = 0.0017). This confirms the efficacy of neoCRT over neoRT in controlling local disease. OS, disease-free survival (DFS), rate of distant metastases, and long-term side effects were not significantly different among the different groups.
A 2013 Cochrane meta-analysis of five major clinical trials reported that although 5-year OS (63.9 % in neoCRT vs. 65.2 % in neoRT; P = 0.58) and DFS (57.5 % in neoCRT vs. 54.9 % in neoRT; P = 0.27) were not statistically different between the two groups, the incidence of LR was significantly lower in the neoCRT group compared to neoRT group (9.4 % vs. 16.5 %; P < 0.001). Although moderate acute toxicity and postoperative complications (P = 0.05) were higher when chemotherapy was added, there was no increase in the postoperative mortality (2.8 % in neoCRT vs. 1.9 % in neoRT; P = 0.17) or anastomotic leak rate (P = 0.81). Finally, neoCRT increased the rate of pathologic complete response (pCR; 11.8 % in neoCRT vs. 3.5 % in neoRT group; P < 0.00001) but did not result in a higher sphincter preservation rate (50.4 % in neoCRT vs. 48.3 % in neoRT; P = 0.32). Based on these results, one can surmise that the major advantage of adding chemotherapy to neoRT is the significant reduction of local recurrence in patients with stage II/III resectable rectal cancer (Table 18.3).
Preoperative Chemoradiotherapy Versus Postoperative Chemoradiotherapy
Whether preoperative chemoradiotherapy (neoCRT) is better than postoperative chemoradiotherapy (postCRT) was the focus of three randomized trials, the Intergroup (INT) 0147, the National Surgical Adjuvant Breast and Bowel Project (NSABP) R-03 [89], and the German Rectal Cancer Study Group (Working Group of Surgical Oncology/Working Group of Radiation Oncology/Working Group of Medical Oncology of the Germany Cancer Society or CAO/ARO/AIO) [90] (Table 18.4). Unfortunately, INT 0147 had to close prematurely because it accrued only 53 patients, while NSABP R-03 accrued 267 patients instead of the intended 900 patients [93]. The latter trial, with a median follow-up of 8.4 years, demonstrated a significantly improved DFS and a trend toward improved OS in favor of the preoperative arm, although there was no improvement in the rate of local control [89].
Table 18.4




Selected trials comparing neoadjuvant chemoradiotherapy versus adjuvant chemoradiotherapy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree