Contemporary surgical management of cancer of the head and neck is the product of the continued application of new oncologic and reconstructive techniques. Patient survival and functional rehabilitation have improved since the mid-1940s, before which orthovoltage radiation was the mainstay of treatment of cancer of the head and neck. With the introduction of modern techniques of anesthesia, antibiotics, blood banking, and new techniques of radical surgery, wide resection of primary cancers of the upper aerodigestive tract and incontinuity neck dissection of regional metastases has resulted in improved cure rates. Thereafter, advances in radiation therapy led to the introduction of “combined therapy.” As an accepted trade-off for improved survival rates, this aggressive approach often resulted in prolonged hospitalization, major functional and cosmetic deficits, and, in many cases, social isolation, as well as the inability to maintain gainful employment.
The development of reconstructive techniques for head and neck surgery did not progress at the same pace as combined therapy for eradication of cancer of the head and neck. In fact, most authors either failed to acknowledge the issue of reconstruction or deemed it unnecessary. Hayes Martin,
1 the father of modern head and neck surgery, wrote:
“Excessive or too frequent resort[ing] to more complicated and technical procedures, such as skin graft for pharyngeal defects, skin graft of the tongue or buccal surface,… bone grafts in mandibular defects, and particularly nerve grafts for [seventh cranial] nerve defects, is not characteristic of the mature or resourceful surgeon.”
Before 1963, most oral and pharyngeal defects were closed primarily, reconstructed with random-pattern skin flaps (such as the nape of neck flap), or reconstructed with tubed, pedicled flaps of skin from the trunk. These flaps rarely matched the tissue requirements of the defect. Furthermore, such repairs were unpredictable and frequently resulted in flap necrosis, salivary fistula, bone or carotid artery exposure, or other complications that led to prolonged hospitalization or the patient’s death.
The previously limited ability of surgeons to resurface mucosal defects of the head and neck improved with the description of the forehead flap by McGregor
2 in 1963 and the deltopectoral flap by Bakamjian
3 in 1965. These well-vascularized, axial-pattern skin flaps permitted more reliable closure of oral and pharyngeal defects at the time of ablative surgery. Although these reconstructive techniques permitted extensive resection to be performed more safely, their limitations soon became apparent. The limited arc of rotation frequently necessitated multistaged, delayed procedures and prolonged hospitalization. The need to perform skin grafts for all but the smallest donor defects contributed to suboptimal aesthetic results. Furthermore, the limitations of the transferred tissue in restoration of function frequently led to permanent impairment of deglutition, articulation, and mastication. Despite their drawbacks, the forehead and deltopectoral flaps were the mainstays of soft tissue reconstruction of head and neck defects for nearly two decades.
The rehabilitation of patients with cancer of the head and neck has been revolutionized since the mid-1970s by the development of advanced reconstructive techniques. Pedicled myocutaneous flaps and free tissue transfers have allowed reliable and safe one-stage primary reconstruction of defects of the upper aerodigestive tract. In the late 1970s and early 1980s, the pedicled pectoralis major myocutaneous flap was popularized and became the predominant method used in reconstruction of the head and neck. Other regional flaps, such as the trapezius and latissimus dorsi flaps, were described for reconstruction of defects of the head and neck region. As clinical experience accumulated, the limitations of pedicled flaps for some reconstructive problems became apparent. These include the limited lengths of the pedicle with restriction of the arcs of rotation, excessive bulk, and donor site morbidities. In addition, the inability of surgeons to reliably transfer vascularized bone for mandibular reconstruction stimulated the search for alternative techniques.
A new approach to transferring tissue became available in 1973 with the advent of microvascular surgery. Subsequently, free tissue transfer rapidly evolved from a reconstructive “last resort” into the preferred method of addressing a variety of complex defects of the head and neck. As new donor sites were discovered and microsurgical techniques were refined, the advantages of free tissue transfer for certain reconstructive problems became apparent. These advantages include the following: (1) superior vascularity of the tissues, resulting in improved tissue survival and wound healing in unfavorable recipient beds; (2) freedom from a limited arc of rotation and length of the vascular pedicle; (3) greater availability, variety, and versatility of donor tissue; and (4) presence of donor sites that are less morbid and conspicuous.
Surgeons are now able to perform more extensive resections with the comfort and confidence of knowing that the available reconstructive procedures can successfully repair the defect in the primary setting and provide the cancer patient with the best opportunity for a rapid functional and cosmetic rehabilitation, as well as prompt initiation of postoperative adjuvant treatments. A good example of the interplay between
reconstructive and ablative surgery is seen in cases affecting the region of the cranial base, where vital structures such as the brain and carotid artery can now be reliably covered and protected with well-vascularized tissue. The ability to separate the cranial cavity from the sinonasal tract has been critical to the advancement of the emerging discipline of cranial base surgery. Despite longer and more technically demanding procedures, the success rate of microvascular free tissue transfers to the head and neck region has continued to improve, approaching 98% and 99%.
4,5 Consequently, free tissue transfer has become an essential part of the comprehensive management of many surgical defects in the head and neck.
This chapter presents our approach to the various problems of reconstruction of defects in the head and neck. Available techniques, indications for clinical application, and functional and aesthetic issues are discussed. Although skin grafts and local flaps are effective for the resurfacing of small mucosal and cutaneous defects, reconstruction of larger defects usually requires transfer of tissue from regional or distant sites. This chapter addresses the latter techniques and also describes the contemporary approach to achieve functional dental restoration. Finally, an approach to the functional assessment of the head and neck cancer patient will be presented in order to provide a framework for assessing the outcomes of surgery and adjuvant therapy and its relationship to the patient’s posttreatment quality of life.
Successful reconstruction requires accurate preoperative assessment and formulation of an individualized treatment plan. Careful consideration of a variety of factors is essential, the most important of these factors is the nature of the defect of the head and neck. Other important considerations include the following: (1) specific histologic features of the tumor, clinical stage, and associated prognosis; (2) age, sex, body habitus, and associated health problems of the patient; (3) available flap donor sites; (4) available recipient vessels; (5) patient compliance with perioperative care, patient expectations, and the psychosocial needs of the patient; and (6) clinical experience and skills of the surgeon. Consequently, a rigid “cookbook,” algorithm-based approach for reconstruction of the head and neck is ill advised. Superior results are seen when the reconstructive team has a wide range of options, which permits the reconstruction to be customized to the individual patient, based on careful consideration of all pertinent tumorand patient-related factors. Hence, just as the overarching approach to contemporary cancer management has stressed individualized treatment strategies, so too has the approach to reconstruction. In addition, it has been our experience that a multidisciplinary approach to the patient’s reconstructive surgery and rehabilitation provides an opportunity for optimal outcomes. This approach begins in the preoperative planning stage and parallels the multidisciplinary approach to the overall cancer management.
GOALS OF RECONSTRUCTION
The primary goal of treatment of cancer of the head and neck is to effect a cure or significant palliation. In addition, every effort should be made to restore the patient to the premorbid level of functioning and quality of life. Advances in radiation and chemotherapy have altered the approach to cancers arising in many subsites of the upper aerodigestive tract, where surgery has been relegated to the salvage setting to eradicate persistent or recurrent cancers. When surgery is used, especially in the oral cavity, it often involves surgical ablation of the primary site and removal of regional nodal metastases. Except in rare cases, some form of reconstruction is necessary. No reconstructive procedure, however elaborately or creatively conceived, should preempt adequate tumor resection nor should such a plan be rigidly adhered to if events during the ablation dictate that an alternative approach will result in a preferred oncologic outcome.
For the reasons outlined above, contemporary reconstruction of the head and neck often takes place in patients who have undergone prior radiation therapy. This is particularly true in the management of complications of radiation therapy such as osteoradionecrosis (ORN) and pharyngoesophageal stenosis.
6,7,8 The reconstructive surgeon must adopt a unique approach in patients with prior radiation therapy to the head and neck region, as there are significant wound healing challenges that can pose a threat to the safety of the surgical undertaking as well as impacting the ultimate functional and aesthetic outcomes.
Successful reconstruction of defects in the head and neck requires that the surgeon define the nature of the defect and create an appropriate strategy to replace the resected tissues with an appropriate substitute.
9 Each subsite of the head and neck presents unique challenges and requires different reconstructive approaches. The major subsites include the cranial base, radical parotidectomy, palatomaxillary, oromandibular, oropharyngeal, and laryngopharyngeal defects. Cutaneous defects of the head and neck may result from management of skin cancer or direct extension to the skin from cancers arising in underlying tissues. Optimal results usually require reconstruction with tissues that simulate the appearance and function of the resected tissue. The tissues required to achieve these goals may include the following: (1) epithelium, to resurface a mucosal or skin defect; (2) muscle, to restore motion or provide coverage of irradiated tissues; and (3) bone, to provide skeletal support. Less frequently, tissues such as fascia and adipose tissue may be required for static suspension or for restoration of contour. Complex defects of the head and neck, such as those resulting from oromandibular and cranial base resection, may require reconstruction with a composite flap. It is in such cases that microvascular free tissue transfer has been most widely used.
The first priority in reconstruction of defects in the head and neck should be safety. It is essential to prevent life-threatening complications, such as carotid blowout, or cerebrospinal fluid (CSF) leak and subsequent meningitis, after resection of the cranial base. The next priority is to return the upper aerodigestive tract to a functional state. Restoration of oral competence, mastication, deglutition, and articulation are the major objectives of reconstruction of the oral cavity following ablative surgery. Maintaining mobility of the tongue, restoring occlusal relationships, preventing trismus, and setting the stage for functional dental rehabilitation are the specific strategies to achieve those goals. Advances in dental prosthetics and the ability of surgeons to reliably transfer vascularized bone have been essential in this regard.
Surgeons should be sensitive to the impact of physical appearance on the patient’s sense of well-being and to the patient’s ability to reintegrate into the society of their family, friends, and work environment. Reconstruction with local tissue is preferred for cutaneous defects because skin adjacent to
the defect provides the optimal match in color and texture. If local tissue is unavailable, the skin quality of potential flaps to be transferred from regional or distant donor sites should be considered. Similarly, when maintenance of bulk in the flap is important aesthetically, the progressive atrophy of denervated muscle should be anticipated. The use of well-vascularized subcutaneous adipose tissue may provide a more effective long-term result. Aesthetic units should be managed as a total entity when possible, especially in resurfacing of defects of the cheek, nose, lips, forehead, and less critically, the neck. To maximize the aesthetic result in the patient with cancer of the head and neck, adjunctive procedures, such as scar revision and flap recontouring, can be performed subsequent to the initial reconstructive effort.
The patient’s age, comorbid conditions, disease status, and motivation may relegate the aesthetic outcome to a secondary concern, whereas functional outcomes are almost always of paramount importance. Defects of the oral cavity and oropharynx, especially those involving the tongue and palate, frequently require more elaborate techniques for the restoration of effective articulation and deglutition. Maintenance of a patent pharyngoesophageal segment and restoration of velopharyngeal competence are vital to achieving optimal functional outcomes. Understanding the function of the ablated tissues and the underlying physiology of the region helps to guide the surgeon in selecting the appropriate strategy for each patient.
PREOPERATIVE PLANNING AND TIMING OF RECONSTRUCTION
Comprehensive management of patients with cancer of the head and neck begins with thorough preoperative planning. For select defects, multidisciplinary planning and implementation of the treatment approach can help to ensure that the best possible outcome is achieved. Patients with cancer typically present with numerous comorbidities and risk factors, including cardiac, renal, pulmonary, cerebrovascular, and hepatic disease. Malnutrition and general debility, secondary to the disease process or to previous surgery, radiation, or chemotherapy, are also common. A thorough initial history, review of systems, and physical examination should provide an evaluation of these risk factors that can be augmented with additional diagnostic studies. An assessment of cardiopulmonary risk factors, especially coronary artery disease, is essential in determining whether a patient is a candidate for a more elaborate reconstructive approach that includes microvascular surgery. Postoperative cardiopulmonary instability can be a threat to the patient and to the reconstructive effort. Mapping of the extent of the primary cancer by a combination of physical examination and radiographic assessment allows the reconstructive surgeon to estimate the probable extent of resection and consideration of the most likely reconstructive approach. In select cases, examination under anesthesia can provide extremely valuable information as to the expected extent of the resection.
The anticipated defect should be classified according to its osseous, soft tissue, and neurologic components. Consideration of the functional region encompassed by the deficit is of far greater importance than a description of the total area or volume of tissue involved. The possible need for coverage of the carotid artery or dura is also discussed. If free tissue transfer is contemplated, potential donor sites and recipient vessels should be evaluated to assess their suitability. Based on all available information, a reconstructive plan is formulated. A second, and even a third, “fallback” option should be considered in the event that the initial plan proves to be unfeasible or fails during the postoperative period.
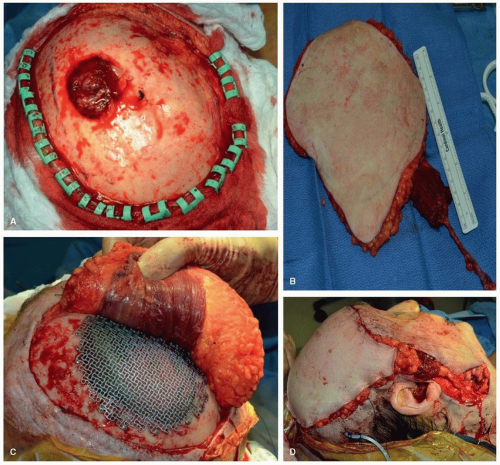
Reconstruction of the maxilla and mandible may be optimized through the use of computer-generated medical models. In select clinical situations, the creation of a medical model can greatly enhance the efficiency and the accuracy of the surgical plan. The most common instances where we have found medical models to be useful are for patients with existing segmental defects of the mandible and maxilla and for patients with tumors that significantly distort the normal bone contour, making fabrication of a rigid fixation plate difficult. The ability of computerized planning to accurately create a surgical model represents a major advance and one that should be anticipated in the preoperative period. The importance of this approach is due to the fact that the computer planning software can reestablish the normal “predisease” shape of the jaw that allows for a more precise plate contouring and a more accurate replication of the patient’s normal facial contour
(Fig. 28.1).
Most reconstructive procedures in the head and neck are optimally performed in one stage at the time of ablation. Primary reconstruction, although lengthening the surgical
procedure, provides an opportunity to introduce the necessary elements of the reconstruction and to ensure the highest likelihood of a safe and successful postoperative recovery. Optimal conditions for reconstruction are present at the completion of tumor resection: (1) the defect is widely exposed; (2) bone and soft tissue requirements are readily and accurately assessed; and (3) potential recipient vessels for microvascular anastomoses, if indicated, are already dissected. Furthermore, surgical margins can be cleared by frozen section pathologic analysis, which permits definitive wound repair. This approach avoids the problems associated with delayed reconstruction, including fibrosis of remaining muscles and contraction of soft tissues within the wound bed. Consequently, primary reconstruction has been shown to provide superior functional and aesthetic results when compared with delayed reconstruction. In addition, the attitude of patients undergoing primary reconstruction is greatly improved when maximal form and function are promptly restored.
Certain reconstructive procedures of the head and neck, however, should be performed in a staged or delayed manner. Multistaged repairs of complex nasal and auricular defects are prime examples. In these instances, the surgeon should plan to perform the fewest number of procedures necessary to obtain optimal results in the shortest possible time. In cases in which tumor-free margins are in question, reconstruction should be delayed until permanent pathologic confirmation is made. Frequently, the functional and cosmetic results of an elaborate reconstructive effort can be maximized by subsequent revisions. These secondary procedures may include scar revision, flap debulking, and placement of endosteal dental implants for dental rehabilitation.
RECONSTRUCTIVE OPTIONS
In general, the least complex method that provides a safe reconstruction while restoring form and function should be selected. The reconstructive surgeon should first consider primary closure or the use of a skin graft. Larger defects usually require alternative methods, such as local, regional, or distant (free) flaps. This logical progression from simple to more complex techniques provides a systematic approach for evaluating whether a given technique satisfies the functional and cosmetic requirements of each defect in the head and neck.
Local Flaps
Local flaps are effective reconstructive alternatives for certain small- to medium-sized defects of the face, neck, and upper aerodigestive tract. When used judiciously, local tissue transfer is aesthetically and functionally superior to the use of more elaborate regional or distant free flaps. The use of tissue adjacent to the defect often provides the best match of skin in terms of its color and texture.
The size and location of the defect and the properties of the available local tissue help to determine whether a local flap is an appropriate reconstructive method. The vascular supply of each local flap is unique and dictates the amount of tissue that can be reliably transferred. Defects of the nose and lips are optimally reconstructed with local muscle-skin flaps, such as forehead, nasolabial, Abbé-Estlander, and Karapandzic flaps. Small intraoral defects can be closed with palatal and buccal mucosal flaps.
10 A more detailed description of the various local flaps is beyond the scope of this chapter.
Regional Flaps
Large defects in the head and neck can be reconstructed with tissues from adjacent regions. Pedicled regional flaps have been used extensively to provide closure of large defects in the neck, face, scalp, oral cavity, and pharynx. They can be classified as fasciocutaneous, such as the deltopectoral flap and the submental island flap, or myocutaneous, such as the pectoralis major, trapezius, and latissimus dorsi flaps. In additional, the temporoparietal fascial flap can be raised as purely a fascial flap or in conjunction with the overlying skin. The temporalis muscle flap is a valuable source of vascularized muscle in the reconstruction of the infratemporal fossa, skull base, and orbitozygomatic defects. Most regional flap procedures can be performed in one stage. However, a “delay” may be instituted to more reliably increase the overall size of the flap. For most cutaneous defects of the neck, the thin, supple quality of the deltopectoral flap is preferred. However, for coverage of the carotid artery or reconstruction of a large oropharyngeal defect, the additional bulk and reliable vascular supply of a myocutaneous flap are advantageous. The arc of rotation is another intrinsic property that must be considered. Only the pedicled latissimus dorsi and lower island trapezius flaps have an extensive arc of rotation that can reliably reach large defects of the scalp. The selection of a specific regional flap depends on the location and size of the defect and the intrinsic properties of the regional flap.
Deltopectoral Flap
The deltopectoral flap is a medially based axial-pattern fasciocutaneous flap of the upper chest that is based on the second and third intercostal, parasternal, perforating branches of the internal mammary artery. The distolateral extent of the flap is determined by the location of the defect relative to the rotational length of the flap; however, distal flap necrosis occurs frequently when the flap is extended onto the shoulder beyond the deltopectoral groove. If greater flap length is desirable, a delay procedure is often necessary to incorporate a random portion of skin over the deltoid muscle.
11The deltopectoral flap is primarily used for resurfacing cutaneous defects of the neck. The introduction of other regional or distant tissue transfer has limited the role of the deltopectoral flap for facial, oral, and pharyngeal reconstruction. Nonetheless, it remains a useful tool for selected reconstructive needs. Although traditionally harvested as a peninsula of skin, it can also be modified as an “island configuration” and tunneled into the neck beneath the skin of the upper chest. This produces a more pleasing contour to the neck and a more favorable reconstructive outcome.
The Submental Island Flap
The submental island flap provides tissue that satisfies the color and texture requirements of the facial skin. It is a reliable and useful flap in reconstruction of the cutaneous defects in the lower and middle thirds of the face. A flap up to 6 to 8 cm in vertical height can be harvested and primary closure achieved, especially in older individuals with older patients with increased skin laxity
(Fig. 28.2). The flap is based on the submental branch of the facial artery, which must be carefully preserved. Potential for injury to this vessel during level IB dissection may limit the use of the flap when supraomohyoid neck dissection is indicated for oncologic reasons. Proximal ligation
of the facial artery and vein with retrograde flow designed can enhance the arc of rotation of the flap.
Myocutaneous Flaps
These axially based flaps have segmental vascular pedicles that enter the deep surface of the muscle, course longitudinally, and send perforating branches through the muscle and subcutaneous tissue to the overlying skin. A large amount of well-vascularized tissue can be transferred in a single stage to reconstruct almost any defect in the head and neck, from the pharyngoesophagus to the lateral skull base.
Despite their widespread use, regional myocutaneous flaps have certain disadvantages. In defects that require thin, pliable skin, the excess bulk of a myocutaneous flap may lead to a less than optimal result. Regional myocutaneous flaps have limited length, skin paddle size, and arc of rotation. The sacrifice of a regional muscle to provide a vascular supply to the overlying skin may result in some degree of functional disability as well as in moderate distortion of the anterior chest or back.
Pectoralis Major Flap. The pectoralis major originates from the medial one-third of the clavicle (cephalic portion), the sternum and cartilages of the upper six ribs (central or sternocostal portion), and the external oblique aponeurosis (caudal portion). The primary vascular supply is from the pectoral branch of the thoracoacromial artery, with a lesser contribution from the lateral thoracic artery.
The pectoral branch of the thoracoacromial artery is visualized on the undersurface of the pectoralis major muscle and enters the muscle medial to the tendon of the pectoralis minor, whereas the lateral thoracic artery courses lateral to the tendon. In most instances, the pectoralis major muscle and the overlying skin are passed over the clavicle into the neck. In rare circumstances, a tunnel deep to the clavicle, created by removing and repositioning a small portion of the clavicular bone may be warranted.
12When first introduced, the pectoralis major flap was used primarily for reconstruction of mucosal defects of the oral cavity and pharynx and cutaneous defects of the neck. As previously mentioned, the pectoralis major flap is still considered the “workhorse” for head and neck reconstruction especially in settings where resources are limited and/or microvascular reconstruction is not available. In places where microvascular reconstruction is available, the pectoralis major flap plays an important role, especially in the setting of salvage surgery. It can be used to provide muscle coverage over the carotid artery or to supplement microvascular reconstruction. The pectoralis major muscle can also obliterate dead space, for example, after mediastinal dissection. The pectoralis major myocutaneous flap is extremely reliable, as indicated by a low incidence of reported complications. The incidence of total flap necrosis has been reported as 1% to 3%.
13 The incidence of partial flap necrosis, as high as 30% in some series, is probably related to the degree of caudal extension of the skin paddle over the rectus sheath. Depending on the patient’s body habitus, the pectoralis flap may be less reliable for more cephalic defects of the face, scalp, and pharynx. Furthermore, the effect of gravity on the bulky pectoralis major muscle may be detrimental,
especially when the flap is placed in an unfavorable recipient bed or when a patient is at risk for compromised wound healing.
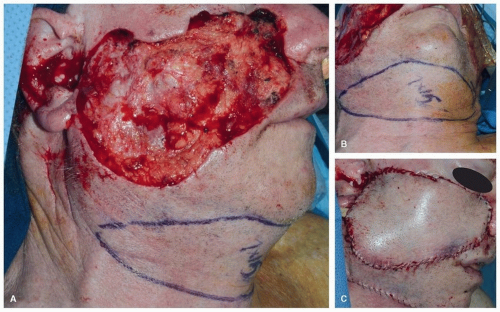
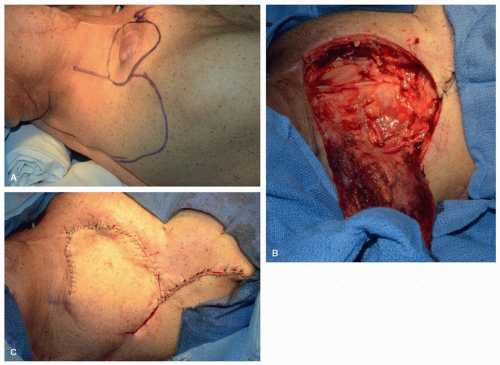
Trapezius Flaps. The trapezius muscle is a broad, thin, triangular muscle that covers most of the upper back and posterior neck. The vascular supply of the trapezius muscle is more complex and variable than that of the other regional myocutaneous flaps used for reconstruction in the head and neck. The dominant blood supply is from the transverse cervical artery (TCA), which consists of a superficial branch and a deep branch. The deep branch of the TCA is also known as the dorsal scapular artery (DSA). Lesser contributions from the occipital artery and the posterior intercostal perforators are also present.
Of the three distinct trapezius flaps, the superior trapezius flap
14 is the most reliable. The primary blood supply is from the paraspinous perforators with some contribution from the occipital artery and the ascending branch of the superficial branch of the TCA. Unlike the other trapezius flaps, its blood supply is unaffected by prior radical neck dissection with sacrifice of the transverse cervical vessels. The superior trapezius flap is used primarily for protection of the carotid artery and for the resurfacing of lateral cervical cutaneous defects
(Fig. 28.3). Because it is superiorly based, the effect of gravity does not cause the flap to pull away from the bed. The donor site usually cannot be closed primarily, and therefore, a skin graft is needed. Also, a dog-ear correction is often required.
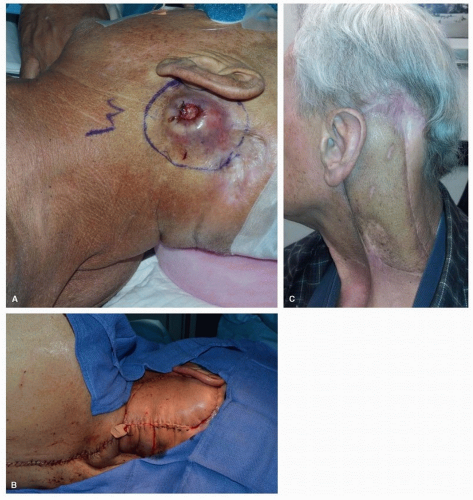
The lateral island trapezius flap
15 is based on the TCA. It can reach the anterior neck, oral cavity, and pharynx in some cases, but its arc of rotation is dependent on favorable vascular anatomy of the transverse cervical system in the posterior triangle of the neck and the degree of mobilization of the TCA and TCV. Because of this variable vascular anatomy, preliminary exploration of the posterior triangle is essential. If the TCA is coursing deep to the brachial plexus, it will be impossible to mobilize the lateral island trapezius flap. Following harvest of this flap, wide undermining should allow primary closure of the donor site defect.
The lower island flap
16 is the most versatile with the greatest arc of rotation of the three trapezius myocutaneous flaps. The skin paddle is designed over the inferior third of the trapezius muscle, between the vertebrae and the scapula. The superior arc of rotation of this flap permits reliable closure of defects in the posterior neck, temporal bone, and scalp
(Fig. 28.4). Less frequently, it has been used for reconstruction in the oral cavity and pharynx. The major disadvantage of the lower island flap is the necessity of placing the patient in the lateral decubitus position for the flap harvest.
Latissimus Dorsi Flap. When all pedicled myocutaneous flaps are considered, the latissimus dorsi flap has the largest potential skin area (25 × 40 cm) available for transfer to the head and neck.
17,18,19,20 Two separate cutaneous paddles may be designed based on the intramuscular bifurcation of the thoracodorsal vascular pedicle for reconstruction of through-andthrough defects. The functional disability that results from the transfer of the latissimus dorsi muscle is reportedly less than that resulting from the use of either the pectoralis or the trapezius muscle.
18 This flap is frequently transferred as a free flap, and details relevant to the anatomy, surgical technique, and clinical application of the latissimus dorsi flap are discussed further in the following section on the Scapular System of Flaps.
Temporoparietal Fascia Flap
The thin and pliable tissue of the temporoparietal fascial flap is useful for lining of defects in the upper face where excess bulk is undesirable. Its location makes it invaluable in the reconstruction of auricular defects, and its thinness and pliability
allow the cartilaginous architecture to be revealed. Variations in flap harvest include incorporation of the forehead or scalp skin and, less frequently, vascularized split calvarium bone. The integrity of the superficial temporal vascular pedicle is essential to ensuring flap viability.
Temporalis Muscle Flap
The temporalis muscle receives its main vascular supply from the anterior and deep temporal artery branches of the internal maxillary artery. It is used in reconstruction of the skull base, the infratemporal fossa, and the maxillectomy defects. Although employed extensively in the past, the use of this muscle in facial reanimation is currently limited to temporalis tendon transfer for suspension of the lower lip. The primary disadvantages of this donor site include a fairly limited reach and the resultant hollowing in the temporal area.
Microvascular Free Tissue Transfer
One of the most important benefits of free tissue transfer is the superior blood supply that maximizes tissue survival and wound healing in unfavorable, contaminated head and neck recipient sites. Thus, it promotes healing despite scarring, radiation damage, and salivary contamination of the recipient bed. The second major benefit relates to the freedom of being able to inset a free flap without being restricted by a limited vascular pedicle, as is common with regional flaps. The skin islands of the pedicled pectoralis major, trapezius, and latissimus flaps are often transferred from the distal and least vascular portions of the territory. Free tissue transfers are more efficient in that the flap can be placed into the defect with less concern for distal flap necrosis. Also, certain recipient sites, in particular the scalp and cranial base, may be beyond the reliable reach of most regional flaps. Even if a regional myocutaneous flap reaches the defect, the effect of gravity on the pedicle may place additional tension on a tenuous suture line.
Another advantage of free flaps is the greater variety and versatility of donor sites. Free tissue transfers such as the scapula megaflap or the iliac crest-internal oblique flap permit the harvesting of multiple tissue paddles based on a single vascular pedicle. Thus, free tissue transfers can be designed to restore more complex defects more precisely than can the tissues from adjacent regional donor sites. The disadvantages of free tissue transfer arise from the complexity of the technique and the increased surgical time required. As with regional pedicled flaps, the color and contour of free flaps in certain cases may not exactly match those of the recipient site. If the patient is a poor surgical risk, a more expedient and less complex technique that uses a regional flap may offer a safer reconstructive alternative.
It is essential that the characteristics of various free tissue transfer approaches be carefully considered
21 (Tables 28.1 and 28.2). Several anatomic areas, including the groin, abdomen, back, and extremities, provide reliable fasciocutaneous, musculocutaneous, and osteomusculocutaneous flaps. Each donor site has inherent advantages and disadvantages. For vascularized, bone-containing free flaps, the amount of bone stock available and the flexibility of the soft tissue component in relation to the bone are important considerations. The morbidity incurred at the donor site following free flap harvest must also be taken into account.
Fasciocutaneous Free Flaps
Fasciocutaneous free flaps provide a segment of skin and subcutaneous adipose tissue of variable size and thickness for reconstruction of a wide variety of frequently encountered defects. Flaps commonly used for head and neck reconstruction include (1) the radial forearm flap, (2) the lateral arm flap, (3) the anterolateral thigh flap, and (4) the scapular and parascapular flaps (see under Scapular System of Flaps). The pliability of these flaps allows for precise anatomic restoration of resected tissue during oral cavity, oropharyngeal, and hypopharyngeal reconstruction. The radial forearm, lateral arm, and anterolateral thigh flaps have the potential for sensory reinnervation, which may be helpful for the rehabilitation of mastication and deglutition in oral cancer patients. Because adipose tissue within revascularized flaps does not atrophy, fasciocutaneous flaps allow for precise and permanent restoration of contour deformities.
Radial Forearm Flap. The radial forearm free flap is a fasciocutaneous flap that is harvested from the volar aspect of the forearm. It provides a large amount of thin, pliable skin that has the potential for sensory reinnervation via the antebrachial cutaneous nerves. Consequently, it has become the free tissue transfer of choice for the resurfacing of oral cavity and oropharyngeal defects.
22 It has also gained considerable popularity for reconstruction of the hypopharynx and cervical esophagus. In select situations, it has also been used to resurface the scalp and a variety of areas of the face, including the cheek, nose, chin, and forehead.
The radial forearm flap has numerous advantages:
It provides a large amount of relatively thin, often hairless, skin that can be folded on itself to conform to nearly any mucosal or cutaneous defect.
It has a long vascular pedicle and vessels of large size, facilitating dissection and revascularization.
Sensation can be restored to the skin paddle by the incorporation of the medial or lateral antebrachial cutaneous nerves.
23,24
The donor site permits simultaneous two-team harvest and dissection under tourniquet control.
A moderate amount of subcutaneous adipose tissue can be left attached to the vascular pedicle, either for protection of the great vessels and the flap’s vascular pedicle or for augmentation of contour deformities, such as those seen following radical neck dissection.
The radial forearm flap can also be used as an osteocutaneous free flap because a 10- to 12-cm-long bone segment encompassing 40% of the circumference of the radius can be harvested with the skin paddle.
25
However, when used as an osteocutaneous flap for mandibular or maxillary reconstruction, the limited bone stock restricts the potential for functional dental restoration.
The disadvantages of the radial forearm free flap are primarily aesthetic. Unless extremely small, the forearm donor defect requires skin grafting. In addition, the color and texture match to facial skin is only fair. A potentially devastating complication of the radial forearm flap is vascular compromise of the hand. To avoid this complication, a preoperative Allen’s test is essential to assess the circulation of the hand. Other complications include insufficient flow to the hand despite a normal Allen’s test, numbness of the hand as a result of trauma
to the superficial branches of the radial nerve, devastating infection resulting in suppurative tenosynovitis, and poor take of the skin graft secondary to failure to preserve the paratenon over the flexor tendons, leading to exposure of the forearm tendons as a result of incomplete healing of the skin graft over the donor defect.
Lateral Arm Flap. Although this flap is harvested most frequently as a fasciocutaneous flap,
26 it can also be raised with a monocortical segment of vascularized humerus, triceps tendon, or brachialis muscle. Alternatively, the flap can be de-epithelialized and used as a vascularized fascia-adipose tissue flap for soft tissue augmentation. Dye injection studies indicate that
the lateral arm flap can incorporate a cutaneous paddle that ranges from 8 × 10 cm to 14 × 15 cm. The width of skin that is harvested with the lateral arm flap is usually limited to 6 to 8 cm or one-third of the circumference of the arm, which is the largest cutaneous defect that can be closed primarily.
In the head and neck region, the lateral arm flap has been used most frequently for facial and intraoral reconstruction.
27,28 For soft tissue augmentation, it is of intermediate thickness relative to the thin radial forearm flap and the thicker anterolateral thigh and scapular system of flaps.
The lateral arm flap has several advantages over other fasciocutaneous flaps commonly employed in head and neck reconstruction. Unlike the radial forearm flap, which requires harvesting of the radial artery, harvesting of the posterior radial collateral artery (PRCA) with the lateral arm flap poses no risk for limb ischemia, and the donor site rarely requires a skin graft. Unlike the scapular fasciocutaneous flap, the lateral arm flap may be harvested with the patient in the supine position, allowing a simultaneous two-team approach.
Relative disadvantages of the lateral arm flap include a linear scar on the lateral aspect of the arm and anesthesia of the forearm as a result of transection of the posterior cutaneous nerve to the forearm. Dissection of the vascular pedicle of the lateral arm flap can be more tedious than harvesting procedures for the radial forearm or scapular fasciocutaneous flaps. This pedicle has an average length of 4 to 8 cm, which limits its application to certain head and neck defects.
Anterolateral Thigh Flap. The anterolateral thigh flap is a fasciocutaneous flap that is harvested from the anterior thigh in the area overlying the septum between the rectus femoris and the vastus lateralis muscles. It is based on the descending branch of the lateral circumflex femoral artery and its venae comitantes. The vascular pedicle can be up to 16 cm in length with large-diameter vessels. Primary closure of the donor site can usually be achieved, even following the harvest of large skin paddles. Sensory reinnervation is possible with incorporation of the lateral cutaneous nerve of the thigh. Due to these features, it has become quite popular for use in reconstruction in the head and neck.
29,30
The anterolateral thigh flap is often used in pharyngoesophageal defects or other large mucosal defects. Its advantages include a large area of skin for harvest and a relatively straightforward dissection with minimal donor site morbidity. Its location allows for an easy two-team approach, and no special positioning is required.
Disadvantages of this flap include excessive flap thickness in obese patients, the potential for hair-bearing skin in men, and the necessity to take a cuff of vastus lateralis muscle in 60% of patients in whom the skin is supplied by perforators that traverse the muscle rather than by a pure septocutaneous route.
31
Myocutaneous Free Flaps
Myocutaneous free flaps are a versatile reconstructive tool for the head and neck surgeon because they provide bulky tissue with reliable vascularity to the overlying skin and offer distinct advantages over regional myocutaneous flaps, namely, a greater versatility in design and the 3-dimensional freedom of maneuverability for flap insetting. Their use is most appropriate for the reconstruction of extensive defects of the tongue, scalp, skull base, and paranasal sinuses. In addition, some free muscle flaps can be reinnervated for reanimation of the paralyzed face. The most commonly used musculocutaneous free flaps include (1) rectus abdominis, (2) gracilis, and (3) latissimus dorsi (see under Scapula System of Flaps).
Rectus Abdominis Flap. A unique feature of the rectus abdominis flap is that a substantial amount of muscle and skin can be harvested. Musculocutaneous perforators are located in the periumbilical region and oriented toward the inferior border of the scapula. Because of these perforators, the skin of a significant portion of the abdomen may be transferred reliably.
32
The rectus abdominis flap is one of the most versatile and commonly performed free tissue transfers in head and neck reconstruction.
33 It is used frequently when bulky soft tissue is required for reconstruction. For example, it is useful following total glossectomy. One of its greatest uses is in skull base reconstruction, especially in an irradiated patient, to prevent a CSF leak and an ascending infection and to provide vascularity to a free calvarial bone graft.
The rectus abdominis flap offers numerous advantages:
Its primary vascular pedicle—the deep inferior epigastric artery and vein (DIEA, DIEV)—is long and of large diameter.
It can be harvested with the patient in the supine position, allowing a two-team approach.
A large amount of tissue can be harvested and primary closure of the donor defect can still be achieved
(Fig. 28.5).
The rich vascularity of the abdominal wall allows great flexibility in the design of the paddles. Multiple skin paddles of varying thickness, based on the periumbilical perforating vessels, can be designed for use in the reconstruction of complex three-dimensional defects. The skin paddles can be oriented in a transverse, a vertical, or an oblique direction.
Donor site morbidity is minimal, as long as the rectus fascia is repaired to prevent formation of a ventral hernia.
The durable anterior rectus fascial sheath and tendinous inscriptions facilitate placement of sutures during insetting of the flap. This allows for a watertight closure and obliteration of dead space, which are critical in the oral cavity and in reconstruction of the cranial base.
34
The major potential disadvantage of the rectus free flap is its excessive bulk, especially in obese patients. This can be corrected by subsequent debulking procedures or by intraoperative modification of the flap’s design. An alternative solution is to harvest the muscle alone or in combination with a variable thickness of subcutaneous tissue. A skin graft can be placed to resurface the muscle if necessary. In any case, postoperative atrophy of the muscle layer should be anticipated.
Gracilis Flap. The gracilis muscle is a long strap-like muscle that arises from the pubic symphysis and ramus and inserts below the knee onto the fibula. The gracilis muscle is favored by many surgeons who perform free tissue transfer for facial reanimation because the muscle’s individual fascicular territories allow the use of smaller muscle units for restoration of specific facial movements. It can also be used as an innervated myocutaneous flap for reconstruction of the tongue or for radical parotidectomy defects when the mimetic facial muscles are resected or cannot be reinnervated.
35,36
Vascularized Bone-Containing Free Flaps
Vascularized, bone-containing free flaps have revolutionized the reconstruction of segmental mandibular and palatomaxillary defects by reliably restoring continuity of bone and soft tissue in the primary setting. The fibula, iliac crest, and scapula all provide vascularized bone of adequate stock to replace the resected segment. All have advantages, limitations, and donor site morbidities, leading to their use in different circumstances. The most important differences relate to the quality, quantity, and reliability of the soft tissue component of the composite flap. Other essential differences include (1) the potential for osseointegration of the bone component
37,38; (2) the length and caliber of the vascular pedicle; (3) the ease of positioning, harvesting, and insetting of the flap; and (4) the potential complications and functional deficits associated with the sacrifice of bone and adjacent soft tissue at the donor site.
Fibula Flap. The principal attribute of the fibula free flap is the exceptional length of bone it provides, making it the only bone-containing free flap that is adequate for total or subtotal mandibular reconstruction.
39 Up to 26 cm of fibula bone can be harvested in the adult male, and the rich periosteal blood supply of the fibula from the peroneal artery allows the creation of multiple wedge-shaped osteotomies for precise contouring of the neomandible or maxilla, without compromise of bone viability
(Fig. 28.6). It is important that the periosteum over the bone to be used is not stripped, and thus, the bone and periosteum are cut together when creating osteotomies.
40 The thick bicortical bone accepts osseointegrated implants for dental rehabilitation.
41
The peroneal artery supplies the fibula and also gives rise to septocutaneous perforators that run in the posterior crural septum to supply the skin of the lateral calf. Because there are variations in the location of the perforators to the skin paddle, it is best to design a long anterior curvilinear incision that will allow exposure to the intermuscular septal perforators along the entire length of the available bone
(Fig. 28.7). The posterior incision can then be modified based on the location of the perforator(s) and dimensions of the soft tissue defect.
42Advantages include simultaneous two-team harvest with the patient in the supine position. Furthermore, sensory reinnervation of the skin paddle is possible through incorporation of the lateral sural nerve into the flap design. Due to the
quality of the bone and soft tissue, it has been used extensively for palatomaxillary reconstruction.
43The main disadvantage of the fibula free flap is the limitation imposed by its soft tissue component. Fasciocutaneous and musculocutaneous perforators of the peroneal artery supply the skin over the lateral calf and permit the harvest of a composite osteocutaneous flap. However, the poor arc of rotation of the skin island relative to bone and its unpredictable pattern of vascularity limit its application to soft tissue reconstruction. Although methods to increase the reliability of the skin paddle have been described,
44 extensive composite oromandibular defects should be reconstructed with an alternative flap, such as the scapula osteocutaneous free flap or the internal oblique-iliac crest composite free flap. Alternatively, a bone-containing free flap can be used in combination with a separate fasciocutaneous free flap or a regional pedicled flap. The presence of atherosclerosis or congenital vascular anomalies of the lower extremity must be identified preoperatively and contraindicate the harvest of the fibula free flap. A preoperative angiogram, CTA or MRA, should be performed to delineate these abnormalities.
The donor site morbidity associated with the fibula osteocutaneous flap is minimal. Two potential complications are injury to the peroneal nerve, which results in foot drop, and instability of the knee or ankle joints. Both of these complications can be avoided provided that the proximal and distal 6 to 8 cm of fibula bone is preserved.
In summary, the osseous and composite osteocutaneous fibula flaps are valuable additions to the available composite flaps used for oromandibular and palatomaxillary reconstruction. The donor site provides the largest length of available bone with limited functional impairment relative to other bone donor sites.
Iliac Crest Flap. The large amount of bone available from the ileum has made it a popular source for nonvascularized bone grafts, corticocancellous chips as well as vascularized bone transfer. The advantages of the ileum as a donor site are numerous. They include (1) the thick bicortical bone stock, which facilitates dental prosthetic rehabilitation with osseointegrated implants; (2) the ability of the donor site scar to be well hidden by conventional undergarments; and (3) the ease of flap harvest by a separate surgical team with the patient in a supine position. In addition, the anterior ileum is similar in shape to the native hemimandible. A total of 10 to 16 cm of bone can be harvested, and osteotomies can be made to reconstruct hemimandibular or angle-to-angle defects. Furthermore, depending on the soft tissue needs of the patient, the iliac crest free flap can be harvested as an osseous flap, an osteocutaneous flap, or a tripartite osteomyocutaneous flap when used in combination with the internal oblique muscle. The iliac crest free flap with the internal oblique muscle has been applied to palatomaxillary reconstruction with good results, allowing placement of osseointegrated implants and closure of the palate with the use of the soft tissue components.
45
The bulkiness, limited pliability, and restricted mobility of the skin paddle relative to the bone, limit the use of the osteocutaneous flap for reconstruction of complex composite defects. Alternatively, the highly mobile, thin, and well-vascularized internal oblique muscle can be used to resurface defects in the oral cavity and pharyngeal mucosa. Similarly, a portion of the muscle can be used to cover the bone graft and reconstruction plate. Skin grafting of the internal oblique muscle is unnecessary because the well-vascularized flap rapidly mucosalizes. The denervated muscle subsequently undergoes atrophy and provides a thin, well-vascularized, and immobile layer of tissue over the mandible.
46The iliac crest free flap has been applied to the reconstruction of a variety of defects in the head and neck. The osteocutaneous flap has been used for reconstruction of the anterior mandible in association with total or subtotal glossectomy defects. In this instance, the iliac bone is placed transversely in the floor of the mouth to support the skin paddle, which is used to reconstruct the tongue. Excellent long-term maintenance of the height of the neotongue has been reported with this flap design.
47 The iliac crest free flap has also been used for reconstruction of skull base and maxillectomy defects.
48The harvest of the iliac crest free flap involves a considerable amount of dissection. Because most of the lower abdominal wall muscles and part of the inguinal ligament are divided, ventral hernia formation is a significant potential risk. Meticulous closure of the donor site, including the use of mesh for select cases, helps to minimize this risk. The patient experiences postoperative hip pain and weakness, but this generally subsides after several weeks. Despite these potential donor site problems, extensive clinical experience with the iliac crest free flap has demonstrated its reliability in achieving functional oromandibular reconstruction for even the most complex composite mandibulectomy defects.
Scapular System of Flaps. Flaps based on the distal ramifications of the subscapular artery provide a wide array of available tissue for reconstruction of defects in the head and neck. These flaps include the scapular and parascapular fascial or fasciocutaneous free flaps, the lateral or medial scapular osteocutaneous free flaps, the serratus anterior flap, the latissimus dorsi muscle or myocutaneous flap, the latissimus dorsi-rib flap, and the serratus anterior-rib flap.
49,50,51,52
After its takeoff from the subscapular artery, the thoracodorsal artery descends along the surface of the serratus muscle for a distance of 9 cm before entering the latissimus dorsi muscle. The latissimus dorsi muscle may be harvested as a pedicled or free muscle flap, a myocutaneous flap, or an osteomyocutaneous flap incorporating a segment of rib. The thoracodorsal artery is consistently accompanied by the thoracodorsal vein and the thoracodorsal nerve, which is the motor nerve to the latissimus dorsi muscle. This neurovascular pedicle has been used to provide reinnervated muscle for total glossectomy reconstruction and facial nerve rehabilitation. It is important to note that the functional advantage of reinnervated muscle in total glossectomy reconstruction has not been demonstrated conclusively.
The angular branch arises from the thoracodorsal vessels or the branch to the serratus anterior and supplies the periosteum of the tip of the scapula. Dissection and inclusion of this branch allows for up to 8 cm of scapular tip to be harvested independently from a separate lateral scapular bone flap based on the circumflex scapular artery. This permits the transfer of two separate scapular bone segments based on a single subscapular vascular pedicle and separated by a 13- to 15-cm arc of rotation.
53 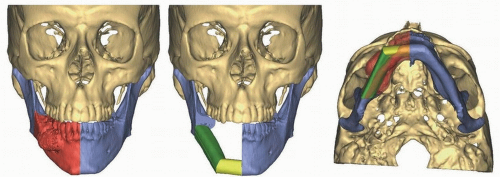


 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access